Pain Descending Pathways: Difference between revisions
No edit summary |
No edit summary |
||
| Line 5: | Line 5: | ||
</div> | </div> | ||
== Introduction == | == Introduction == | ||
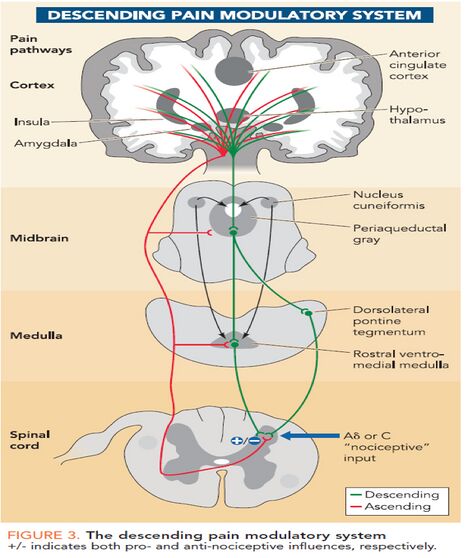
Once a pain signal from the ascending pathway reaches the somatosensory cortex, it triggers the descending pain modulatory system. The goal of this pathway is to allow the organism to function enough to respond to the pain source by reducing the pain signal through neuronal inhibition ie the ''"top down"'' modulation of pain. It begins in the periaqueductal gray (PAG), an area of grey matter in the midbrain that is involved in a descending pain control pathway. The periaqueductal gray, or PAG, receives pain information via the spinomescencephalic tract and processes the nociceptive information and relays it to the rostral ventral medulla (RVM). These neurons in the RVM then send a signal down the spinal cord and activate the endogenous opiate system to suppress pain.<ref>Neuroscientifically Challenged PAG Available:https://neuroscientificallychallenged.com/glossary/periaqueductal-gray (accessed 18.4.2022)</ref><ref name=":0">Tufts Available: Opioid peptides<nowiki/>https://sites.tufts.edu/opioidpeptides/pathways-and-receptors/classical-opioid-signaling/ (accessed 18.4.2022)</ref>[[Image:Descending-inhibitory-pathway.jpg|border|center|558x558px|alt=]]Our body produces natural (endogenous) opioids, which comprise endorphins, dynorphins, and enkephalins. On a molecular level, both these and synthetic opioids inhibit pain signals by interfering with intracellular signaling and preventing ascending pain-causing neurons from sending action potentials. <ref name=":0" /> Opioids have actions at two sites, the presynaptic nerve terminal and the postsynaptic neuron. The postsynaptic actions of opioids are usually inhibitory. The presynaptic action of opioids is to inhibit neurotransmitter release, and this is considered to be their major effect in the nervous system<ref>NPS medicine Opioids Available: https://www.nps.org.au/australian-prescriber/articles/opioids-mechanisms-of-action<nowiki/>(accessed 18.4.2022)</ref>. | |||
Opioid receptors are located in the: brain (highest densities found in the thalamus, the PAG, and the RVM); spinal cord (high density in the dorsal horn region) and; peripheral organs, eg heart, blood vessels, kidneys, sympathetic ganglia, and the adrenal medulla)<ref>Decaillot F, Abul-Husn NS, Devi L. Opioid and Opioid-like Receptors.Available: https://www.sciencedirect.com/science/article/pii/B9780080552323601035 (accessed 8.4.2022)</ref><br> | |||
== Why is the System Useful? == | |||
= | Evidence for pain modulalatory mechanisms were first recorded by Beecher<ref name="Beecher">Beecher HK. Pain in men wounded in battle. Ann Surg. 1946;123(1):96-105</ref>. Beecher, a physician serving the US Army during World War II, observed as many as three quarters of badly wounded soldiers reported none to only moderate pain and did not require pain relief medication. According to his report the men were alert and responsive and the injuries were not trivial, including compound fractures and penetrating wounds. This led him to the conclusion that "strong emotions" block pain. This clearly opposess the classical Cartesian view where pain was considered to be a hard-wired system that passively transmitted noxious inputs to the brain. | ||
It is now generally accepted that the experience of pain does not solely rely on noxious inputs, but many variables interplay with the experience, including memory, mood, environment, attention and expectation. Ultimatly, this means the resultant pain experienced to the same sensory input can vary considerably<ref name="Bingel">Bingel U, Tracey I. Imaging CNS modulation of pain in humans. Physiolohy 2008;23:371-380</ref>. It is the brain's job to weigh all the information and decide whether creating pain is the most appropriate response. This provides a necessary '''survival function''' since it allows the pain experience to be altered according to the situation rather than having pain always dominate<ref name="Bingel" />. | |||
== Implications for Physiotherapists == | == Implications for Physiotherapists == | ||
| Line 26: | Line 21: | ||
Thirdly, <span style="line-height: 1.5em;"> manual techniques such as [[Manual Therapy|joint mobilisations]], manipulations have been proposed to activate the system and significantly contribute to their therapeutic effects<ref name="Wright">Wright A. Hypoalgesia post manipulative therapy: a review of a potential neurophysiological mechanism. Man Ther 1995;1(1), 11-16</ref>. </span><span style="line-height: 1.5em;">Noxious stimuli can activate the system</span><ref name="Yaksh">Yaksh TL, Elde RP. Factors governing release of methionine enkephalin-like immunoreactivity from mesencephalon and spinal cord of the cat in vivo. J.Neurophysiol 1981;46 (5), 1056-1075</ref><ref name="Fields 2">Fields HL, Basbaum AL. Central Nervous System mechanisms of pain modulation, In: Wall PD, Melzack R. Textbook of pain, 4th ed.Churchill Livingstone, Edinburgh; 1999</ref> <span style="line-height: 1.5em;">and this </span><span style="line-height: 19.9200000762939px;">can help explain why manual</span><span style="line-height: 1.5em;"> techniques that may elicit some pain (to some degree) can be helpful to reduce pain overall. </span><span style="line-height: 1.5em;"> This knowledge can aid the physiotherapist with careful selection and use of techniques with a </span>''"top down"''<span style="line-height: 1.5em;"> philosphy, freeing them from selecting interventions based merely on proposed local tissue reponses such as inhibiting reflex muscle contraction, reducing intra-articular pressure and reducing the level of joint afferent activity</span><ref name="Zusman">Zusman M. Spinal manipulative therapy: a review of some proposed mechanisms, and a new hypothesis. Aust J. Physiotherapy 1986;32(2),89-99</ref> | Thirdly, <span style="line-height: 1.5em;"> manual techniques such as [[Manual Therapy|joint mobilisations]], manipulations have been proposed to activate the system and significantly contribute to their therapeutic effects<ref name="Wright">Wright A. Hypoalgesia post manipulative therapy: a review of a potential neurophysiological mechanism. Man Ther 1995;1(1), 11-16</ref>. </span><span style="line-height: 1.5em;">Noxious stimuli can activate the system</span><ref name="Yaksh">Yaksh TL, Elde RP. Factors governing release of methionine enkephalin-like immunoreactivity from mesencephalon and spinal cord of the cat in vivo. J.Neurophysiol 1981;46 (5), 1056-1075</ref><ref name="Fields 2">Fields HL, Basbaum AL. Central Nervous System mechanisms of pain modulation, In: Wall PD, Melzack R. Textbook of pain, 4th ed.Churchill Livingstone, Edinburgh; 1999</ref> <span style="line-height: 1.5em;">and this </span><span style="line-height: 19.9200000762939px;">can help explain why manual</span><span style="line-height: 1.5em;"> techniques that may elicit some pain (to some degree) can be helpful to reduce pain overall. </span><span style="line-height: 1.5em;"> This knowledge can aid the physiotherapist with careful selection and use of techniques with a </span>''"top down"''<span style="line-height: 1.5em;"> philosphy, freeing them from selecting interventions based merely on proposed local tissue reponses such as inhibiting reflex muscle contraction, reducing intra-articular pressure and reducing the level of joint afferent activity</span><ref name="Zusman">Zusman M. Spinal manipulative therapy: a review of some proposed mechanisms, and a new hypothesis. Aust J. Physiotherapy 1986;32(2),89-99</ref> | ||
== References == | == References == | ||
Revision as of 02:08, 18 April 2022
Original Editor - Adrian Mallows.
Top Contributors - Adrian Mallows, Lucinda hampton, Jo Etherton, Admin and Lauren Lopez
Introduction[edit | edit source]
Once a pain signal from the ascending pathway reaches the somatosensory cortex, it triggers the descending pain modulatory system. The goal of this pathway is to allow the organism to function enough to respond to the pain source by reducing the pain signal through neuronal inhibition ie the "top down" modulation of pain. It begins in the periaqueductal gray (PAG), an area of grey matter in the midbrain that is involved in a descending pain control pathway. The periaqueductal gray, or PAG, receives pain information via the spinomescencephalic tract and processes the nociceptive information and relays it to the rostral ventral medulla (RVM). These neurons in the RVM then send a signal down the spinal cord and activate the endogenous opiate system to suppress pain.[1][2]
Our body produces natural (endogenous) opioids, which comprise endorphins, dynorphins, and enkephalins. On a molecular level, both these and synthetic opioids inhibit pain signals by interfering with intracellular signaling and preventing ascending pain-causing neurons from sending action potentials. [2] Opioids have actions at two sites, the presynaptic nerve terminal and the postsynaptic neuron. The postsynaptic actions of opioids are usually inhibitory. The presynaptic action of opioids is to inhibit neurotransmitter release, and this is considered to be their major effect in the nervous system[3].
Opioid receptors are located in the: brain (highest densities found in the thalamus, the PAG, and the RVM); spinal cord (high density in the dorsal horn region) and; peripheral organs, eg heart, blood vessels, kidneys, sympathetic ganglia, and the adrenal medulla)[4]
Why is the System Useful?[edit | edit source]
Evidence for pain modulalatory mechanisms were first recorded by Beecher[5]. Beecher, a physician serving the US Army during World War II, observed as many as three quarters of badly wounded soldiers reported none to only moderate pain and did not require pain relief medication. According to his report the men were alert and responsive and the injuries were not trivial, including compound fractures and penetrating wounds. This led him to the conclusion that "strong emotions" block pain. This clearly opposess the classical Cartesian view where pain was considered to be a hard-wired system that passively transmitted noxious inputs to the brain.
It is now generally accepted that the experience of pain does not solely rely on noxious inputs, but many variables interplay with the experience, including memory, mood, environment, attention and expectation. Ultimatly, this means the resultant pain experienced to the same sensory input can vary considerably[6]. It is the brain's job to weigh all the information and decide whether creating pain is the most appropriate response. This provides a necessary survival function since it allows the pain experience to be altered according to the situation rather than having pain always dominate[6].
Implications for Physiotherapists[edit | edit source]
Knowledge of the descending pain modulatory system and its components can help physiotherapists in several ways. Firstly, it helps physiotherapists explain why the amount of pain a patient is experiencing does not neccesarily relate to the amount of tissue damage they have sustained[5]. Physiotherapists can educate their patients about the role of the descending pain modulatory system and how the central nervous sytem weighs all the information before deciding if a pain experience is the most approriate action for survival. Neuroscience education has been shown to be effective in several studies[7][8][9][10] [11][12].
Secondly, knowledge of the anatomy (see above) involved in the descending pain modulatory system can help physiotherapists utilise management strategies to that access and activate the system. These could include adding distractions to exercises and perfoming exercises in different emotional states and or in different environments.
Thirdly, manual techniques such as joint mobilisations, manipulations have been proposed to activate the system and significantly contribute to their therapeutic effects[13]. Noxious stimuli can activate the system[14][15] and this can help explain why manual techniques that may elicit some pain (to some degree) can be helpful to reduce pain overall. This knowledge can aid the physiotherapist with careful selection and use of techniques with a "top down" philosphy, freeing them from selecting interventions based merely on proposed local tissue reponses such as inhibiting reflex muscle contraction, reducing intra-articular pressure and reducing the level of joint afferent activity[16]
References[edit | edit source]
References will automatically be added here, see adding references tutorial.
- ↑ Neuroscientifically Challenged PAG Available:https://neuroscientificallychallenged.com/glossary/periaqueductal-gray (accessed 18.4.2022)
- ↑ 2.0 2.1 Tufts Available: Opioid peptideshttps://sites.tufts.edu/opioidpeptides/pathways-and-receptors/classical-opioid-signaling/ (accessed 18.4.2022)
- ↑ NPS medicine Opioids Available: https://www.nps.org.au/australian-prescriber/articles/opioids-mechanisms-of-action(accessed 18.4.2022)
- ↑ Decaillot F, Abul-Husn NS, Devi L. Opioid and Opioid-like Receptors.Available: https://www.sciencedirect.com/science/article/pii/B9780080552323601035 (accessed 8.4.2022)
- ↑ 5.0 5.1 Beecher HK. Pain in men wounded in battle. Ann Surg. 1946;123(1):96-105
- ↑ 6.0 6.1 Bingel U, Tracey I. Imaging CNS modulation of pain in humans. Physiolohy 2008;23:371-380
- ↑ Moseley G Combined physiotherapy and education is efficacious for chronic low back pain. Australian Journal of Physiotherapy 2002; 48:297–302
- ↑ Louw A, Louw Q, Crous LCC. Preoperative Education for Lumbar Surgery for Radiculopathy. South African Journal of Physiotherapy. 2009;65(2):3-8.
- ↑ Moseley, GL. A randonmised controlled trial of intensive neurophysiology education in chronic low back pain. Clin J Pain 2002;20:324-330
- ↑ Meeus MJ. Pain physiology education improves pain beliefs in patients with chronic fatigue syndrome compared with pacing and self-management education:a double-blind randomised controlled trial. Arch Phys Med Rehabil 2010;91:1153-1159
- ↑ Clarke CL. Pain Neurophysiology education for the management for the management of individuals with chronic low back pain: systematic review and meta-analysis. Manual Therapy 2011;16:544-549
- ↑ Louw A. The effect of neuroscience education on pain and disability, anxiety, and stress in chronic musculoskeletal pain. Archives of Physical Medicine and Rehabilitation 2011;92:2041-2056
- ↑ Wright A. Hypoalgesia post manipulative therapy: a review of a potential neurophysiological mechanism. Man Ther 1995;1(1), 11-16
- ↑ Yaksh TL, Elde RP. Factors governing release of methionine enkephalin-like immunoreactivity from mesencephalon and spinal cord of the cat in vivo. J.Neurophysiol 1981;46 (5), 1056-1075
- ↑ Fields HL, Basbaum AL. Central Nervous System mechanisms of pain modulation, In: Wall PD, Melzack R. Textbook of pain, 4th ed.Churchill Livingstone, Edinburgh; 1999
- ↑ Zusman M. Spinal manipulative therapy: a review of some proposed mechanisms, and a new hypothesis. Aust J. Physiotherapy 1986;32(2),89-99