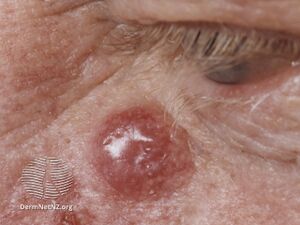
Merkel Cell Carcinoma
Introduction[edit | edit source]
Merkel Cell Carcinoma (MCC) is a rare and aggressive skin cancer typically seen on the facial skin and extremities of elderly Caucasians and immunocompromised people. This neuroendocrine carcinoma accounts for less than 1% of all cutaneous malignancies and is associated with UV exposure and infection with the Merkel cell polyomavirus (MCPyV). It frequently metastasizes to draining lymph nodes and distant organs, including liver, bone, pancreas, lung, and brain.[1][2][3][4]
Epidemiology[edit | edit source]
The worldwide incidence of MCC varies from 0.1 to 1.6 cases per 100,000 people annually with Australia recording the highest rate ranging from 0.82 to 2.5 [1][3][5]. It is then followed by New Zealand (0.88–0.96)[6][7] and United States (0.66–0.79)[1][8][9].
European countries such as France, Spain and Scotland report relatively similar incidence rates of 0.25 to 0.35 while Scandinavian countries have less incidence rates: 0.19 in Sweden and 0.12 in Finland. [1]
Risk factors of MCC[edit | edit source]
Predisposing factors for MCC are as follows:[1]
- Old age. The median age at diagnosis is documented to be 77 years with the highest incidence observed among people over 85 years old.
- UV exposure. MCC is strongly associated with lower latitudes and high UV radiation indexes and the tumour occurs preferentially on sun-exposed skin. Also, there is a 100-fold increased risk of developing MCC in patients treated with psoralen + UVA.
- White skin type. MCC is very rare in dark-skinned patients, whether they be Black, Hispanic or Asian.
- Male sex. The incidence of MCC is more than 2.5 times greater in men compared to women in nearly all documented studies.
- Immunosuppression. Approximately 6–12% of all patients with MCC are immunosuppressed.
Aetiology and Pathological Process[edit | edit source]
Two distinct aetiologies of MCC:[4]
- Viral form of MCC (Merkel cell polyomavirus)
- Causes at least 60% of all MCC
- Clonal integration of Merkel cell polyomavirus DNA into the tumour genome with persistent expression of viral T antigens
- Broad exposure to this ubiquitous virus is known to occur early in life, but higher antibody levels in older individuals suggest viral reactivation with increased age[3]
- Two oncogenic antigens: large T (LT) and small T (ST). LT has been more extensively studied. Following incorporation of the virus into the genome of the host cell, a truncated LT antigen, with loss of its viral replicative capacity but retention of its antigenic and oncogenic roles, is produced. By binding of truncated LT to the Rb protein and by indirect ST-mediated inhibition of p53, these tumour suppressor pathways are disabled, promoting oncogenesis.[3]
- Nonviral form of MCC
- Caused by UV damage leading to highly mutated genomes
- Tumorigenesis in these cases also involves disruption of the Rb and p53 tumour suppressor pathways, but by different means. In this instance, recurrent inactivating mutations in the TP53 and RB1 genes promote oncogenesis.[3]
Both forms of MCC are similar in presentation, prognosis, and response to therapy.
Clinical Presentation[edit | edit source]
The most common sites of MCC are the sun-exposed areas of head and neck (29–43.9%) and the extremities (36.9–45%), whereas less than 5–10% of MCCs develop on partially sun-protected areas (abdomen, thighs and hair-bearing scalp) or highly sun-protected areas (buttocks). Extra-cutaneous sites are rarely affected. [1]
Clinically, MCC is manifested by a rapidly growing, painless, erythematous/violaceous nodule or plaque.[10]
AEIOU acronym for MCC clinical features:[11]
- A = asymptomatic
- E = expanding rapidly
- I = immune suppression
- O = older than50 years of age
- U=UV-exposed site.
Diagnostic Procedures[edit | edit source]
MCC is frequently misdiagnosed initially which can lead to delay in diagnosis. It may be confused with an inflammatory lesion or a benign tumour. In many other cases, MCC is misinterpreted as another malignant tumour. A clinically useful recommendation is that any nodule with non-specific morphology, lack of tenderness and fast-growing should be biopsied rather than monitored. [1]
Physical examination[edit | edit source]
Assessment of the primary tumour and documentation done by a dermatologist for MCC:[2]
- Light photography
- Palpation of regional lymph node basins
- Inspection of the entire skin surface by a dermatologist.
Dermatoscopy[edit | edit source]
Dermatoscopic images of MCC reveals:[1]
- Predominant red colour corresponding either to numerous vessels or generalised erythema
- Milky-red or pink structureless colour. It might be seen either as a pink background or as smaller roundish areas (milky red areas or globules or clods).
- Several morphologic types of vessels may be present, including dotted, glomerular, arborising and linear irregular vessels. Usually, more than one morphologic type of vessel co-exist, resulting in the so-called polymorphous vascular pattern, although lesions with monomorphous vessels have also been described.
- White areas are also frequently described.
Histological Diagnosis[edit | edit source]
Immunohistochemistry is required to distinguish MCC from its mimics. Depending on size and location, tissue sampling in suspicious lesions should be accomplished by punch, incisional or excisional biopsy. MCC generally consists of a solid nodular lesion in the dermis and subcutis[1]
On haematoxylin eosin stains, the tumour typically exhibits: [1]
- sheets and nests of uniform small round blue undifferentiated cells with scant cytoplasm
- a ‘salt and pepper’ chromatin pattern
- large lobulated nucleoli
- high mitotic rate
- occasional necrotic cells.
In clinical practice, a typical histology complemented with positive CK-20 and negative TTF-1 immunostaining is usually considered sufficient for the diagnosis of MCC.[1]
Management / Interventions[edit | edit source]
Surgery and Adjuvant Radiotherapy[edit | edit source]
Radical excision is generally considered the first-line treatment for MCC. Wide resection margins, between 1 cm and 3 cm depending on localization, are recommended to not only obtain free resection margins, but also to include potential small satellite lesions close to the primary tumour.[2]
In virtually all cases, postoperative radiotherapy is indicated because it reduces the regional recurrence risk and improves the 3-year disease-specific survival from 48% to 76%[2]
Definitive Radiotherapy[edit | edit source]
MCC is very radiosensitive and definitive radiation monotherapy is used as an alternative to surgery for patients who are poor surgical candidates or for those in whom surgery would result in significant functional compromise. Currently, there are no prospective clinical studies comparing surgery with or without adjuvant radiotherapy with radiotherapy alone. [2]
Immunotherapy[edit | edit source]
MCC is a very immunogenic tumour which offers good prospects for immunotherapy. New developments in MCC studies suggest that immunotherapy can cause long-lasting responses in a significant proportion of patients with recurrent or metastatic MCC.[1][2]
Chemotherapy[edit | edit source]
A comprehensive systematic review in 2017 of chemotherapy regimens in patients with advanced MCC suggests that chemotherapy has no durable response.[12] Additionally, chemotherapy is associated with a high risk of toxicity, particularly in elderly patients who frequently have impaired liver and kidney function as well as a limited bone marrow reserve. Adverse effects of aggressive chemotherapy include myelosuppression, sepsis, fatigue, alopecia, nausea/vomiting and renal injury. For these reasons, chemotherapy is only to be considered as a palliative strategy after failure or contraindication to immunotherapy. [1]
Multidisciplinary Approach[edit | edit source]
A multidisciplinary approach to clinical decision-making is vital in MCC due to its complex and aggressive nature as well as the lack of research to guide the management of immune deficiency. Multidisciplinary tumour board consultations (dermatologist, surgeon and radiotherapist) for patients with advanced MCC are needed to consider all options for the management of advanced MCC cases.[1]
Differential Diagnosis[edit | edit source]
Differential Diagnosis of MCC:[1][3]
- non-neuroendocrine small cell undifferentiated carcinoma
- small cell melanoma
- cutaneous lymphoma
- poorly differentiated carcinoma metastatic to the skin (e.g. small cell lung cancer)
- Ewing sarcoma
- neuroblastoma
- leukaemia cutis
References[edit | edit source]
- ↑ 1.00 1.01 1.02 1.03 1.04 1.05 1.06 1.07 1.08 1.09 1.10 1.11 1.12 1.13 1.14 Gauci ML, Aristei C, Becker JC, Blom A, Bataille V, Dreno B, Del Marmol V, Forsea AM, Fargnoli MC, Grob JJ, Gomes F. Diagnosis and treatment of Merkel cell carcinoma: European consensus-based interdisciplinary guideline–Update 2022. European Journal of Cancer. 2022 Aug 1;171:203-31.
- ↑ 2.0 2.1 2.2 2.3 2.4 2.5 Zwijnenburg EM, Lubeek SF, Werner JE, Amir AL, Weijs WL, Takes RP, Pegge SA, van Herpen CM, Adema GJ, Kaanders JH. Merkel cell carcinoma: New trends. Cancers. 2021 Mar 31;13(7):1614.
- ↑ 3.0 3.1 3.2 3.3 3.4 3.5 Walsh NM, Cerroni L. Merkel cell carcinoma: A review. Journal of Cutaneous Pathology. 2021 Mar;48(3):411-21.
- ↑ 4.0 4.1 DeCaprio JA. Molecular pathogenesis of Merkel cell carcinoma. Annual Review of Pathology: Mechanisms of Disease. 2021 Jan 24;16:69-91.
- ↑ Garbutcheon-Singh K, Curchin D, McCormack C, Smith S. Trends in the incidence of merkel cell carcinoma in Victoria between 1986 and 2016. InAUSTRALASIAN JOURNAL OF DERMATOLOGY 2019 May 1 (Vol. 60, pp. 40-40). 111 RIVER ST, HOBOKEN 07030-5774, NJ USA: WILEY.
- ↑ Lee Y, Chao P, Coomarasamy C, Mathy JA. Epidemiology and survival of Merkel cell carcinoma in New Zealand: a population‐based study between 2000 and 2015 with international comparison. Australasian Journal of Dermatology. 2019 Nov;60(4):e284-91.
- ↑ Robertson JP, Liang ES, Martin RC. Epidemiology of Merkel cell carcinoma in New Zealand: a population‐based study. British Journal of Dermatology. 2015 Sep 1;173(3):835-7.
- ↑ Fitzgerald TL, Dennis S, Kachare SD, Vohra NA, Wong JH, Zervos EE. Dramatic increase in the incidence and mortality from Merkel cell carcinoma in the United States. The American surgeon. 2015 Aug;81(8):802-6.
- ↑ Paulson KG, Park SY, Vandeven NA, Lachance K, Thomas H, Chapuis AG, Harms KL, Thompson JA, Bhatia S, Stang A, Nghiem P. Merkel cell carcinoma: Current US incidence and projected increases based on changing demographics. Journal of the American Academy of Dermatology. 2018 Mar 1;78(3):457-63.
- ↑ Becker JC, Stang A, DeCaprio JA, Cerroni L, Lebbé C, Veness M, Nghiem P. Merkel cell carcinoma. Nature reviews Disease primers. 2017 Oct 26;3(1):1-7.
- ↑ Heath M, Jaimes N, Lemos B, Mostaghimi A, Wang LC, Peñas PF, Nghiem P. Clinical characteristics of Merkel cell carcinoma at diagnosis in 195 patients: the AEIOU features. Journal of the American Academy of Dermatology. 2008 Mar 1;58(3):375-81.
- ↑ Nghiem P, Kaufman HL, Bharmal M, Mahnke L, Phatak H, Becker JC. Systematic literature review of efficacy, safety and tolerability outcomes of chemotherapy regimens in patients with metastatic Merkel cell carcinoma. Future oncology. 2017 Jun;13(14):1263-79.