Peripheral Arterial Disease
Original Editors - Students from Glasgow Caledonian University's Cardiorespiratory Therapeutics Project.
Top Contributors - Victoria Michaud, Lucinda hampton, Kim Jackson, Admin, Adam Vallely Farrell, Chris Seenan, Vidya Acharya, 127.0.0.1, Michelle Lee and WikiSysop
Introduction[edit | edit source]
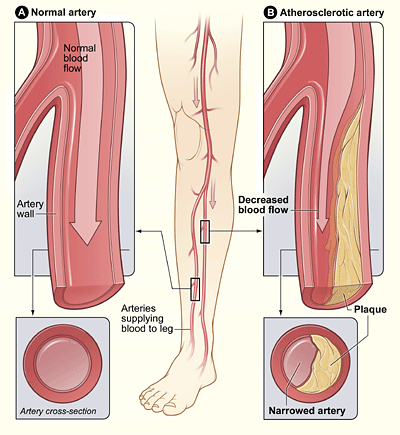
Peripheral artery disease is a common type of cardiovascular disease, which affects 236 million people across the world. It happens when the arteries in the legs and feet become clogged with fatty plaques through a process known as atherosclerosis.
While some people with this disease experience no symptoms, the most classic symptoms are pain, cramps, numbness, weakness or tingling that occurs in the legs during walking – known as intermittent claudication. These problems affect around 30% of people with peripheral artery disease. Intermittent claudication is more common in adults over 50, men and people who smoke.[1]
The management of PAD varies depending on the disease severity and symptom status. Treatment options for PAD include lifestyle changes, cardiovascular risk factor reduction, pharmacotherapy, endovascular intervention, and surgery.[2]
Epidemiology[edit | edit source]
Prevalence: 12-14%, 20% of the over 70s in Western populations[3].
Smoking increases the risk of developing PAD fourfold and has the greatest impact on disease severity. Compared to non-smokers, smokers with PAD have shorter life spans and progress more frequently to critical limb ischemia and amputation. Additional risk factors for PAD include diabetes, hyperlipidemia, hypertension, race, and ethnicity.
Etiology[edit | edit source]
Peripheral artery disease is usually caused by atherosclerosis. Other causes may be inflammation of the blood vessels, injury, or radiation exposure.[2]
Risk factors: Smoking, Hypertension, Diabetes, High cholesterol, Increasing age (especially after reaching 50 years of age), Family history of peripheral artery disease, Heart disease or Stroke, High levels of homocysteine (a protein component that helps build and maintain tissue).[2]
Prognosis[edit | edit source]
If PAD is left untreated it does not inevitably lead to amputation. At five years from diagnosis most patients with claudication have stable or improved symptoms.[4] Asymptomatic disease is identified as a marker of sedentary lifestyle rather than less severe disease and outcomes are similar to those with claudication. As much as 25% of symptomatic patients will need some form of intervention, but less than 5% of those will progress to critical limb ischaemia. [5] The risk of amputation is 1-3.3% and all-cause mortality is 20% within five years from diagnosis of PAD. [5] The risk of limb amputation is 30% in patients with critical limb ischaemia and five year all-cause mortality is 50%. [5] Mortality rates for all patients that require leg amputation are twice as high when compared to those without amputation. Patients presenting with diabetes are at a greater risk of amputation or dying when compared to non-diabetic patients with PAD. [6]
History[edit | edit source]
The most characteristic symptom of PAD is claudication which is a pain in the lower extremity muscles brought on by walking and relieved with rest.
- Although claudication has traditionally been described as cramping pain, some patients report leg fatigue, weakness, pressure, or aching.
- Symptoms during walking occur in the muscle group one level distal to the artery narrowed or blocked by PAD. eg Patients with aortoiliac artery occlusive disease have symptoms in the thigh and buttock muscles, patients with femoropopliteal PAD have symptoms in their calf muscles.
- Some patients with mild or moderate PAD rarely sustain a walking pace that increases the blood flow requirement of the lower extremity muscles. By being physically inactive, these patients avoid the supply-demand mismatch that triggers claudication symptoms.
- Other patients with PAD have muscle discomfort when they walk but fail to report these symptoms because they attribute them to the natural consequences of aging.
Patients with severe PAD can develop ischemic rest pain.
- These patients do not walk enough to claudicate because of their severe disease.
- They complain of burning pain in the soles of their feet that is worse at night. They cannot sleep due to the pain and often dangle their lower leg over the side of the bed in an attempt to relieve their discomfort. The slight increase in blood flow due to gravity temporarily diminishes the otherwise intractable pain.
The video below is a good summary of the basics of PAD
Investigations[edit | edit source]
A tool used to gain a diagnosis of PAD is Ankle Brachial Pressure Index (ABI), a simple and inexpensive test that measures the ratio between blood pressure in the legs to the blood pressure in the arms[8]. The lower the pressure in the legs illustrates that PAD is present. An ABI of 0.9- 1.0 is normal, 0.70-0.89 is a mild disease, 0.40- 0.69 is a moderate disease, and less than .40 is a severe PAD[8]. When measuring for ABI, make sure the patient is calm and in a rested position [9]. It is also important to assess individuals if they have diabetes, non-healing wounds on their legs and feet, unexplained pain in their peripherals, and check for femoral and popliteal pulses[9].
Other investigations that are commonly used in the assistance of a diagnosis of PAD are Blood pressure, Electrocardiography, Full blood count, Urea and electrolytes, Random blood glucose or HBA1C, Serum cholesterol, Thrombophilia screen in patients less than 50 years old.
Clinical Manifestations[edit | edit source]
According to NICE:[9]
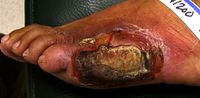
- Non-healing wounds on legs or feet
- Unexplained leg pain
- Pain on walking that resolves when stopped
- Pain in foot at rest made which worsens with elevation
- Ulcers
- Gangrene
- Dry skin
- Cramping
- Aching
Medical Management[edit | edit source]
One method of treating PAD is to reduce cardiovascular risk factors by quitting smoking, managing diabetes mellitus, treating dyslipidemia and hypertension [8]. Another method is to treat PAD symptoms to improve quality of life through pharmacotherapy, exercise rehabilitation program, revascularization, thrombolysis and surgical procedures [8]. The current NICE clinical guidelines on Cardiovascular disease have identified several key aspects in the management of PAD in the primary care setting. It identifies that all patients should receive the following before a referral is made to secondary care.[10] These include;
Risk Factor Modification[edit | edit source]
Smoking cessation therapy[edit | edit source]
Patients with PAD that continue to smoke persistently have worse outcomes. A 2016 AHA ( American Heart Association) statement was updated to include that patients with PAD are now strongly advised to avoid second-hand smoke.[11] When compared with former smokers, they have a greater risk of amputation and their chance of surviving 5 years post diagnosis is halved when compared to non-smokers.[12] It is important that patients are made aware of this association with smoking and the benefits of smoking cessation. A combination of behavioural counselling with medication has been shown to increase the proportion of successful attempts at quitting when compared to standard care.[13] Cessation of smoking may help in preventing further declines in symptoms.
HBA1C control (target value <48 mmol/mol)[edit | edit source]
Numerous studies have shown that an improvement in glycemic control in patients with diabetes reduces the risk of microvascular complications, but has little effect on the risk of amputation.[14] NICE recommend reaching a target HBA1C level of <48 mmol/mol for all patients with diabetes. [10]
Blood pressure control (target value <140/90 mm Hg- for patients <80 years old)[edit | edit source]
Management of hypertension lowers a patients cardiovascular risk. Ramipril is recommended as first line therapy in guidelines worldwide.[15]
Antiplatelet medicine[edit | edit source]
Clopidogrel (or aspirin) 75 mg lifelong - An RCT of patients with atherosclerotic vascular disease showed that clopidogrel 75 mg was significantly better than aspirin 325 mg for prevention of vascular complications at a mean follow up of 1.9years.[16] When clopidogrel is contraindicated, aspirin is an acceptable alternative. Warfarin is most commonly reserved for patients with limb ischaemia due to arterial emboli.
Statins[edit | edit source]
Atorvastatin lifelong - A meta- analysis of 12 observational studies reported that statin therapy plays a role in reducing all-cause mortality and the incidence of stroke in those with PAD. NICE guidelines recommend reducing non- HDL cholesterol concentration in patients with PAD by 40%. [10]
Symptom Control[edit | edit source]
Supervised exercise therapy for 3 months - NICE recommends that a supervised exercise programme is offered to all patients where applicable consisting of 2hrs a week for a 3month period. [10] They report that it is more cost effective than either unsupervised exercise or angioplasty. Despite this, it is estimated that up to 70% of clinical commission groups in the UK fail to provide this service.
Physiotherapy Management[edit | edit source]
The least invasive and most appropriate treatment for PAD conducted by Physiotherapists would be by prescribing an exercise program. The recommended parameters of physical exercise are a 6 month program of 30-35 minutes walking sessions at a frequency of 3-5 times a week at near-maximal pain tolerant.
Supervised exercise programs have proved to have better results than unsupervised exercise programs. An updated Cochrane review 2018 reports that the original version of this review was released in 2006, prescribed exercise therapy consisted mostly of “go home and walk” advice. However, the compelling evidence now suggests that "Evidence of moderate and high quality shows that SET (supervised exercise programs) provides an important benefit for treadmill‐measured walking distance (MWD and PFWD) compared with HBET (home-based exercise programs) and WA (walking advice) respectively."[17]
Even for clients having invasive therapies exercise is important A 2018 Cochrane review comparing mono invasive therapies (monotherapies) to supervised exercise programs (SET) with invasive therapies, concluded " that exercise is a complication-free treatment, it appears to offer significant improvements in patients walk distances with a combination of both SET and intervention offering a superior walking outcome to monotherapy in those requiring invasive measures."[18]
A 2018 review of the best exercise prescription for PAD summarised their findings thus
- Supervised treadmill exercise improves treadmill walking performance in patients with PAD.
- Supervised treadmill exercise has greater benefit on treadmill walking performance than home-based walking exercise.
- Home-based walking exercise interventions that involve behavioral techniques are effective for functional impairment in people with PAD and improve the 6-min walk distance more than supervised treadmill exercise.
- Upper and lower extremity ergometry improve walking performance in patients with PAD and improve peak oxygen uptake.
- Lower extremity resistance training can improve treadmill walking performance in PAD, but is not as effective as supervised treadmill exercise.[19]
The optimal exercise program for PAD recommended by the American Heart Association states the following
Exercise Prescription for Supervised Exercise Treadmill Training in Patients With Claudication[edit | edit source]
- Modality Supervised Treadmill Walking
- Intensity 40%–60% maximal workload based on baseline treadmill test or workload that brings on claudication within 3–5 min during a 6-MWT
- Session duration 30–50 min of intermittent exercise; goal is to accumulate at least 30 min of walking exercise
- Claudication intensity Moderate to moderate/severe claudication as tolerated
- Work-to-rest ratio Walking duration should be within 5–10 min to reach moderate to moderately severe claudication followed by rest until pain has dissipated (2–5 min)
- Frequency 3 times per week supervised
- Program duration At least 12 wk
- Progression Every 1–2 wk: increase duration of training session to achieve 50 min. As individuals can walk beyond 10 min without reaching prescribed claudication level, manipulate grade or speed of exercise prescription to keep the walking bouts within 5–10 min
- Maintenance Lifelong maintenance at least 2 times per week
Based on currently available evidence. Exercise prescription should be individualized to each patient as tolerated. 6-MWT indicates 6-minute walk test. [20]
A recent research study showed that Nordic walking training improved the gait pattern of patients with PAD remarkably and caused a significant increase in the absolute claudication distance and total gait distance. The combined training of Nordic walking with the isokinetic resistance training of the lower extremities muscles (NW + ISO) increased the amplitude of the general center of gravity oscillation to the greatest extent. However, only treadmill training had little effect on the gait pattern. Hence, Nordic walking can be used to rehabilitate patients with PAD as a form of gait training[21].
Outcome Measures[edit | edit source]
Prevention[edit | edit source]
According to Warren[22] there are several methods one can prevent PAD. Firstly, help change the patient's lifestyle by educating them on the risk factors and the effects PAD. If the patient smokes cigarettes, it is important to address the issue and promote cessation. Those who consume a high fat diet have a higher chance of being diagnosed with PAD, thus one should encourage a reduced fat diet as a strong prevention method. Along with diet, it is important to live an active lifestyle. By being active and working up to the general standards of physical activity per week will allow a decrease in weight along with a decrease in risk of PAD.
Conclusions[edit | edit source]
Highlights from the 2016 AHA advice regarding PAD management
- Patients with peripheral artery disease (PAD) should be on a program of guideline-directed medical therapy (including antiplatelet drugs that thin blood and statins to lower cholesterol) and should participate in a structured exercise program.
- Restoring blood flow to the legs through vascular procedures is appropriate for many patients with severe symptoms due to PAD.
- Eliminating exposure to all tobacco – including second-hand smoke – is highly recommended for patients with PAD.[11]
Resources[edit | edit source]
- Peripheral Vascular Disease, NHS Choices
- Peripheral Arterial Disease, Circulation Foundation
- Peripheral Artery Disease, Mayo Clinic
References[edit | edit source]
- ↑ The Conversation Walking can relieve leg pain in people with peripheral artery disease Available: https://theconversation.com/walking-can-relieve-leg-pain-in-people-with-peripheral-artery-disease-151240(accessed 6.6.2021)
- ↑ 2.0 2.1 2.2 Zemaitis MR, Boll JM, Dreyer MA. Peripheral arterial disease. StatPearls [Internet]. 2020 Jul 6.Available :https://www.ncbi.nlm.nih.gov/books/NBK430745/ (accessed 6.6.2021)
- ↑ Radiopedia PAD Available:https://www.ncbi.nlm.nih.gov/books/NBK430745/ (accessed 6.6.2021)
- ↑ Leng GC, Lee AJ, FOWKERS FG, WHITEMAN M, Dunbar J, Housley E, Ruckley CV. Incidence, natural history and cardiovascular events in symptomatic and asymptomatic peripheral arterial disease in the general population. International journal of epidemiology. 1996 Dec 1;25(6):1172-81.
- ↑ 5.0 5.1 5.2 Crawford F, Welch K, Andras A, Chappell FM. Ankle brachial index for the diagnosis of lower limb peripheral arterial disease. Cochrane Database Syst Rev2016;9:CD010680.pmid:27623758
- ↑ Jude EB, Oyibo SO, Chalmers N, Boulton AJ. Peripheral arterial disease in diabetic and nondiabetic patients: a comparison of severity and outcome. Diabetes care. 2001 Aug 1;24(8):1433-7.
- ↑ American Heart Association PAD What is it? Available from: https://www.youtube.com/watch?v=XTSgpiPqIbk (last accessed 7.9.2019)
- ↑ 8.0 8.1 8.2 8.3 Mahameed, AA, Bartholomew, JR, Disease of Peripheral Vessels. In: Topol, EJ, editor. Textbook of Cardiovascular Medicine. 3rd ed. New York: Lippincott Williams & Wilkins, 2007, p.1531-1537
- ↑ 9.0 9.1 9.2 NICE National Institute for Health and Care Excellence. Lower limb peripheral arterial disease: diagnosis and management, 2012. https://www.nice.org.uk/guidance/cg147/chapter/guidance#management-of-intermittent-claudication (accessed 9 May 2015)
- ↑ 10.0 10.1 10.2 10.3 National Institute for Health and Care Excellence. Cardiovascular disease: risk assessment and reduction, including lipid modification (clinical guideline CG181). 2017
- ↑ 11.0 11.1 Newsroom. New peripheral artery disease guidelines emphasize medical therapy and structured exercise 13.11. 2016 Available from: https://newsroom.heart.org/news/x-new-peripheral-artery-disease-guidelines-emphasize-medical-therapy-and-structured-exercise (last accessed 7.9.2019)
- ↑ Lassila R, Lepäntalo M. Cigarette smoking and the outcome after lower limb arterial surgery. Acta chirurgica Scandinavica. 1988;154(11-12):635-40.
- ↑ van de Graaf RC, van Schayck OC. Helping people to give up smoking; efficacy and safety of smoking cessation interventions. Nederlands tijdschrift voor geneeskunde. 2017;161:D1131.
- ↑ Adler AI, Stevens RJ, Neil A, Stratton IM, Boulton AJ, Holman RR. UKPDS 59: hyperglycemia and other potentially modifiable risk factors for peripheral vascular disease in type 2 diabetes. Diabetes care. 2002 May 1;25(5):894-9.
- ↑ Norgren L, Hiatt WR, Dormandy JA, Nehler MR, Harris KA. Inter-Society Consensus for the management of Peripheral Arterial Disease (TASC II) Eur J Vasc Endovasc Surg 33. S1–S75. 2007.
- ↑ National Institute for Health and Care Excellence. Clinical knowledge summaries: Peripheral arterial disease. 2015
- ↑ Hageman D, Fokkenrood HJ, Gommans LN, van den Houten MM, Teijink JA. Supervised exercise therapy versus home‐based exercise therapy versus walking advice for intermittent claudication. Cochrane Database of Systematic Reviews. 2018(4). Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6513337/ (last accessed 6.9.2019)[1]
- ↑ Aherne T, McHugh S, Kheirelseid EA, Lee MJ, McCaffrey N, Moneley D, Leahy AL, Naughton P. Comparing supervised exercise therapy to invasive measures in the management of symptomatic peripheral arterial disease. Surgery research and practice. 2015;2015.Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4639651/ (last accessed 6.9.2019)
- ↑ McDermott MM. Exercise rehabilitation for peripheral artery disease: a review. Journal of cardiopulmonary rehabilitation and prevention. 2018 Mar;38(2):63. Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5831500/ (last accessed 6.9.2019)
- ↑ Diane Treat-Jacobson, Mary M. McDermott, Ulf G. Bronas et al.Optimal Exercise Programs for Patients With Peripheral Artery Disease: A Scientific Statement From the American Heart Association. AHA Journal Vol. Circulation.130 No.4 Available from: https://ahajournals.org/doi/10.1161/CIR.0000000000000623 (last accessed 7.9.2019)
- ↑ W Dziubek, M Stefańska, K Bulińska, K Barska Journal of Clinical …, 2020 - mdpi.comEffects of Physical Rehabilitation on Spatiotemporal Gait Parameters and Ground Reaction Forces of Patients with Intermittent Claudication
- ↑ Warren, E. Ten things the practice nurse can do about peripheral arterial disease. Practice Nurse 2013; 43; 12: 14-18.