Atypical Presentation of Covid in the Elderly
Top Contributors - Ewa Jaraczewska, Lucinda hampton, Tarina van der Stockt, Wanda van Niekerk, Kim Jackson, Jess Bell, Oyemi Sillo, Olajumoke Ogunleye and Aminat Abolade
Introduction[edit | edit source]
The COVID-19 pandemic shows how vulnerable elderly persons are and that the low capacity of the immune system leads to a fatal outcome. Quick implementation of preventive and therapeutic strategies was not possible due to the worldwide nature of COVID. Protection of those at risk who already carried the load of other health issues was limited.
COVID in the Elderly[edit | edit source]
The novel severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) is the aetiology of a new type of viral pneumonia, the Corona Virus Disease 19 (COVID-19).[1] It affects people of all ages everywhere in the world, but a majority of deaths from this disease occur in the elderly. The study shows that in the group of individuals 65 years and older, the most susceptible are those suffering two or more comorbidities. Furthermore elderly with a history of cardiovascular disease, diabetes mellitus, chronic obstructive pulmonary disease, malignancy and chronic kidney disease are the most at risk of dying from COVID.[2] Additional factors include disabilities, cognitive and mood disorders, polypharmacotherapy, social isolation, and nutritional deficits often present in the residents of long-term care facilities.[3]
Infection and Elderly[edit | edit source]
The immune system is responsible for overcoming infections due to the production of IgG anti-virus antibodies. The effectiveness of the immune system can be influenced by several factors but one of the problems leading to its poor responsiveness may be nutritional deficits present in the elderly. It can lead to severe inflammatory disease affecting the heart, lungs, kidneys and vascular system.[1]
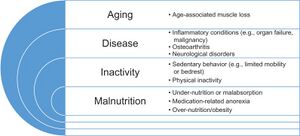
Inflammaging is an elevated inflammatory response level frequently described in the elderly population and caused by 3 geriatric conditions: malnutrition, sarcopenia and frailty.[6]
Nutritional Deficits in Elderly[edit | edit source]
According to the study completed in the European hospitals, residential care and community settings[3] nutritional deficits in the elderly can be caused by reduced dietary intake in addition to age-related problems such as malabsorption, increased nutrient losses and augmented metabolic demands.[1] Malnutrition caused by low intake of food high with vitamins, minerals and proteins directly affect the body's immune responses:
- Low intake of food high with vitamins, especially:
- Vitamin A has an important role in the development and functioning of the immune system, increases the efficacy of T-cell-based viral vaccines[7]
- Vitamin B affects cell and immune system function and energy metabolism. Its deficiency leads to inflammation[8]
- Vitamin C promotes antimicrobial activity and production of the antibody. Tends to be depleted during infections resulting in coagulopathy[9]
- Vitamin D reduces the risk of infections, assist in immune responses[10]
- Vitamin E stimulates the T-cell function.[11]
- Low intake of food high with minerals, especially:
- Low protein intake: leads to wasting syndrome[3]
The risk of malnutrition can be assessed using the Mini Nutritional Assessment.[14] This tool can identify if the individual is well-nourished, is at risk for malnutrition or is malnourished.
Sarcopenia[edit | edit source]
Sarcopenia is a disease that originates at the cellular level. Faulty metabolism can be a causing factor, and symptoms include decreased muscle strength and muscle mass. The progressive cellular processes lead to poor outcomes in an individual's strength, mobility, and functional capacity.[16][17] It is considered one of the risk factors for COVID-19, and at the same time, hospitalisation with COVID-19 infection may lead to the disease.
Clinically patients with sarcopenia demonstrate compromised function of the respiratory system, immunological system, and metabolic system among others.[18] Definition for this clinical presentation was revised in 2018 by the European Working Group on Sarcopenia in Older People (EWGSOP2) and is now used internationally for diagnostic purposes.[19]
Frailty[edit | edit source]
Frailty is a COVID risk factor and the person with the syndrome of frailty must present with three or more symptoms:[20]
- unintentional weight loss (10 lbs in the past year)
- self-reported exhaustion
- weakness (grip strength)
- slow walking speed
- low physical activity.
A person with two or fewer deficits listed above is considered in the pre-frail stage and one displaying no deficits is identified as robust.[6]
Due to reduction in 'physiological reserve affecting immune function and adaptation to acute stressors'[6] frailty is considered high risk for mortality, institutionalization, falls, and hospitalisation.[20]
Typical Presentation of COVID[edit | edit source]
The European Centre for Disease Prevention and Control (ECDC) conducted the study on clinical characteristics of COVID-19.[22] The most common symptoms included:
- headache
- loss of smell
- nasal obstruction
- cough
- asthenia (lack of energy, physical weakness)
- myalgia (muscle aches and pain)
- rhinorrhoea ( nasal discharge)
- gustatory dysfunction (taste disturbance)
- soar throat
- fever
Atypical Presentation of COVID in the Elderly[edit | edit source]
With age‐related changes in immunity, COVID may have an atypical presentation in the elderly population. [23][24]
1. Hypoactive Delirium[edit | edit source]
There are 3 motor subtypes of delirium: hypoactive, hyperactive and mixed category.[25] Hypoactive delirium is the most common subtype of delirium in the geriatric population.[26] The symptoms include lethargy, confusion, and apathy.[27] Because of the patients' behaviour with no signs of agitation, irritation, anger or aggression, they are often wrongly diagnosed with depression or dementia.[26] Hypoactive delirium is associated with systemic infection aetiologies and has a lower overall burden of delirium symptoms than the other motor subtypes,[28] but has been linked to increased risk of mortality.[29][30]
Assessment tools[edit | edit source]
Diagnostic tools to determine a patient's status include standardised testing and information received from the medical record as well as reports from the patient's care team: nursing staff and the family. [28]
Standardised tests:
- Diagnostic and Statistical Manual of Mental Disorders (DSM IV)
- Short Form of the Informant Questionnaire on Cognitive Decline in the Elderly (Short IQCODE)
- Delirium Motor Subtype Scale (DMSS-4)
- Delirium Aetiology Checklist (DEC)
- Delirium Rating Scale Revised-98 (DRS R98)
Clinical Management[edit | edit source]
Management of Hypoactive Delirium includes pharmacological and non-pharmacological approaches.[31]
Non-pharmacological treatment:
- Reorientation
- Living room (scheduled activity with occupational therapist, interaction with other patients)
- Orientation box (clock, diary, information leaflet, radio, TV, music)
- Circadian rhythm (healthy sleep-wake cycle)
- Family participation (family staying at night, room filled with family photos, personal items, pillow, blanket)
- Psychosocial
- Delirium consultation (consultation by a specialist: nurse practitioner, geriatrician, psychiatrist)
- Delirium observation screening score (delirium severity measurement: 13 observations)
2. Falls[edit | edit source]
Syncope, near syncope, or non-mechanical falls are atypical features of COVID.[32] The aetiology of syncope or near syncope fall can be cardiogenic and non-cardiogenic including:
- history of cardiovascular disease
- supplemental oxygen requirement
- gastrointestinal symptoms
- elevated troponin level
The consequences of falls are one of the main causes of disability among the elderly population leading to a reduction in the quality of life, loss of independence and limited social functioning.[33]
Assessment Tools[edit | edit source]
Holistic, multidisciplinary assessment should be considered when evaluating elderly patients with COVID who are at risk for falls and should include:
- fall risk assessment
- evaluation of motor functions
- assessment of cognitive impairment
- mental performance assessment.
Clinical Management[edit | edit source]
Key principles for clinical management of patients with fall risk are:
- education and support for caregivers
- close surveillance to ensure patients' adherence to pharmacological treatment[34]
- providing access to nutritious food, social and mental health support and information to maintain patients emotional well-being[35]
- early planning of post-discharge care.
3. Anorexia of ageing[edit | edit source]
Anorexia of ageing is a loss of appetite due to the ageing process[36], and it can lead to :
- decrease oral intake
- weight loss
- increased risk of malnutrition,
- sarcopenia
- frailty.[36]
It is a condition present among the elderly living in all environments. It affects patients admitted to acute care hospitals the most (42%). 30% of individuals living in the care home and 22% of community-dwelling elderly individuals have been diagnosed with anorexia of ageing.[37] Pathogenesis of the anorexia of ageing has a direct relationship with changes occurring in three "regulators" of appetite: physiological signalling, hedonism and external cues. [36]
Anorexia was diagnosed in 8.4% of the elderly population with COVID-19, and the mortality rate due to malnutrition was high.[38]
Assessment Tools[edit | edit source]
The standardised tools for assessing appetite are not yet available.[36]
The following are the most commonly used instruments for appetite assessment:[39]
- Self-assessment
- Subjective Assessment of Appetite
- Visual Analogue Scale (VAS)
- Functional Assessment of Anorexia/Cachexia Therapy (FAACT) score
- Anorexia Questionnaire (AQ)
- 4-item Simplified Nutritional Appetite Questionnaire (SNAQ)[36]
- Mini Nutritional Assessment
Clinical Management[edit | edit source]
When managing an individual with poor appetite medical causes needs to be ruled out first. Medical conditions known to affect appetite are:
- Chronic heart disease
- Chronic pulmonary disease
- Chronic pain disease
- Acute inflammatory illness
- Medication side effects
- Swallowing deficits (dysphagia).[36]
With no medical causes identified, there is a lack of an evidence-based treatment approach to manage anorexia of ageing. It is suggested that the treatment plan should include:
- nutritional recommendations
- oral nutritional supplements (ONS)
- psychological support
- family-based therapy.
4. Fatigue[edit | edit source]
Patients with COVID often complain about fatigue. This symptom is one of the most common problems affecting 44-69.6% of individuals with COVID.[40] Factors causing fatigue can be biological, social, behavioural, cognitive and emotional.[41] Others include immune system alteration (immune dysregulation).
Chronic Fatigue Syndrome (CFS) is a problem with a lack of endurance that is persistent and lasts over six months.
Assessment Tools[edit | edit source]
Fatigue can be assessed using various tests and scales. Chalder Fatigue Scale (CFQ-11) is a validated tool where patients responses are measured on a Likert scale.[42] Pattern and character of fatigue must be evaluated in addition to exercise tolerance and cognitive exertion.
Common tests include routine haematology and biochemistry with full blood count, urea and electrolytes, thyroid function tests, liver function tests, bone profile, erythrocyte sedimentation rate and vitamin B12.[41]
Clinical Management[edit | edit source]
Lack of evidence-based management strategy for COVID fatigue necessitates the use of treatment methods developed for the management of chronic fatigue syndrome.[41] This approach includes:
- educating the patient
- improving quality of rest
- choosing activities that do not exacerbate symptoms
- learning energy conservation and relaxation techniques
- learning pacing techniques
- incorporating cognitive behavioural therapy (CBT).
References[edit | edit source]
- ↑ 1.0 1.1 1.2 Bencivenga L, Rengo G, Varricchi G. Elderly at time of COronaVIrus disease 2019 (COVID-19): possible role of immunosenescence and malnutrition. GeroScience 2020, 42; 1089–1092.
- ↑ Emami A, Javanmardi F, Pirbonyeh N, Akbari A. Prevalence of Underlying Diseases in Hospitalized Patients with COVID-19: a Systematic Review and Meta-Analysis. Arch Acad Emerg Med. 2020 Mar 24;8(1):e35.
- ↑ 3.0 3.1 3.2 Leij-Halfwerk S, Verwijs MH, van Houdt S, Borkent JW, Guaitoli PR, Pelgrim T, Heymans MW, Power L, Visser M, Corish CA, de van der Schueren MAE; MaNuEL Consortium. Prevalence of protein-energy malnutrition risk in European older adults in the community, residential and hospital settings, according to 22 malnutrition screening tools validated for use in adults ≥65 years: A systematic review and meta-analysis. Maturitas. 2019 Aug;126:80-89.
- ↑ Dr. Maria van Kerkhove. WHO's Science in 5 on COVID-19. Available from: http://www.youtube.com/watch?v=_1s7__6CjtM [last accessed 29/10/2021]
- ↑ ABC 10 News. In-Depth Why COVID-19 hits older people hard. Available from: http://www.youtube.com/watch?v=ul0nw5iAuKM [last accessed 29/10/2021]
- ↑ 6.0 6.1 6.2 Lengelé L, Locquet M, Moutschen M, Beaudart C, Kaux JF, Gillain S, Reginster JY, Bruyère O. Frailty but not sarcopenia nor malnutrition increases the risk of developing COVID-19 in older community-dwelling adults. Aging Clin Exp Res. 2021 Oct 23:1–12.
- ↑ Huang Z, Liu Y, Qi G, Brand D, Zheng S. Role of vitamin A in the immune system. J Clin Med. 2018;7:258
- ↑ 8.0 8.1 Shakoor H, Feehan J, Al Dhaheri AS, Ali HI, Platat C, Ismail LC, Apostolopoulos V, Stojanovska L. Immune-boosting role of vitamins D, C, E, zinc, selenium and omega-3 fatty acids: Could they help against COVID-19? Maturitas. 2021 Jan;143:1-9.
- ↑ Carr AC. A new clinical trial to test high-dose vitamin C in patients with COVID-19. Crit Care. 2020;24:133.
- ↑ Jakovac H. COVID-19 and vitamin D-Is there a link and an opportunity for intervention? Am J Physiol Endocrinol Metab. 2020;318:E589.
- ↑ Lee GY, Han SN. The role of vitamin E in immunity. Nutrients. 2018;10.
- ↑ Cherayil BJ. Iron and immunity: immunological consequences of iron deficiency and overload. Arch Immunol Ther Exp (Warsz). 2010 Dec;58(6):407-15.
- ↑ Tam M, Gómez S, González-Gross M, Marcos A. Possible roles of magnesium on the immune system. Eur J Clin Nutr. 2003 Oct;57(10):1193-7.
- ↑ Vellas B, Guigoz Y, Garry PJ, Nourhashemi F, Bennahum D, Lauque S, Albarede JL. The Mini Nutritional Assessment (MNA) and its use in grading the nutritional state of elderly patients. Nutrition. 1999 Feb;15(2):116-22.
- ↑ Prof Janice Thompson. Busting myths about COVID-19 and nutrition. Available from: http://www.youtube.com/watch?v=_yBx0GVO9vo [last accessed 29/10/2021]
- ↑ Tarantino U, Piccirilli E, Fantini M, Baldi J, Gasbarra E, Bei R. State of Fragility Fractures Management during the COVID-19 Pandemic. J Bone Joint Surg Am. 2015 Mar 4; 97(5):429-37.
- ↑ Moreira VG, Perez M, Lourenço RA. Prevalence of sarcopenia and its associated factors: the impact of muscle mass, gait speed, and handgrip strength reference values on reported frequencies. Clinics (Sao Paulo). 2019 Apr 8;74:e477.
- ↑ Wang PY, Li Y, Wang Q. Sarcopenia: An underlying treatment target during the COVID-19 pandemic. Nutrition. 2021 Apr;84:111104.
- ↑ Cruz-Jentoft AJ, Bahat G, Bauer J, Boirie Y, Bruyère O, Cederholm T, Cooper C, Landi F, Rolland Y, Sayer AA, Schneider SM, Sieber CC, Topinkova E, Vandewoude M, Visser M, Zamboni M; Writing Group for the European Working Group on Sarcopenia in Older People 2 (EWGSOP2), and the Extended Group for EWGSOP2. Sarcopenia: revised European consensus on definition and diagnosis. Age Ageing. 2019 Jan 1;48(1):16-31. doi: 10.1093/ageing/afy169. Erratum in: Age Ageing. 2019 Jul 1;48(4):601.
- ↑ 20.0 20.1 Fried LP, Tangen CM, Walston J, Newman AB, Hirsch C, Gottdiener J et al.Frailty in Older Adults: Evidence for a Phenotype. The Journals of Gerontology 2001: Series A 56(3): M146–M157.
- ↑ Dr Ken Rockwood speaks about frailty and COVID-19. Available from: http://www.youtube.com/watch?v=UT_T2I3p5GI [last accessed 29/10/2021]
- ↑ European Centre for Disease Prevention and Control. Available from https://www.ecdc.europa.eu/en/covid-19/latest-evidence/clinical (accessed 28 Oct 2021).
- ↑ Soysal P, Kara O. Delirium as the first clinical presentation of the coronavirus disease 2019 in an older adult. Psychogeriatrics. 2020 Sep;20(5):763-765.
- ↑ Rawle M, Bertfield D, Brill S. (2020). Atypical presentations of COVID‐19 in care home residents presenting to secondary care: A UK single centre study. AGING MEDICINE 2020. https://onlinelibrary.wiley.com/doi/10.1002/agm2.12126. (Accessed 29 Oct 2021)
- ↑ Lipowski ZJ. Transient cognitive disorder in the elderly. Am J Psychiatry 1982;140:1426–36.
- ↑ 26.0 26.1 Soysal P, Ahmet TI.Hypoactive delirium caused by pulmonary embolus in an elderly adult. J Am Geriatr Soc. 2014 Mar;62(3):586-7.
- ↑ Meagher DJ, Leonard M, Donnelly S, Conroy M, Adamis D, Trzepacz PT. A longitudinal study of motor subtypes in delirium: relationship with other phenomenology, aetiology, medication exposure and prognosis. J Psychosom Res. 2011 Dec;71(6):395-403.
- ↑ 28.0 28.1 Glynn K, McKenna F, Lally K, et al. How do delirium motor subtypes differ in phenomenology and contributory aetiology? a cross-sectional, multisite study of liaison psychiatry and palliative care patients. BMJ Open 2021;11:e041214.
- ↑ Yang FM, Marcantonio ER, Inouye SK, et al. Phenomenological subtypes of delirium in older persons: patterns, prevalence, and prognosis. Psychosomatics 2009;50:248–54.
- ↑ Jackson TA, Wilson D, Richardson S, et al. Predicting outcome in older hospital patients with delirium: a systematic literature review. Int J Geriatr Psychiatry 2016;31:392–9.
- ↑ van Velthuijsen EL, Zwakhalen SMG, Mulder WJ, Verhey FRJ, Kempen GIJM. Detection and management of hyperactive and hypoactive delirium in older patients during hospitalization: a retrospective cohort study evaluating daily practice. Int J Geriatr Psychiatry. 2018 Nov;33(11):1521-1529.
- ↑ Chen T, Hanna J, Walsh EE, Falsey AR, Laguio-Vila M, Lesho E. Syncope, Near Syncope, or Nonmechanical Falls as a Presenting Feature of COVID-19. Ann Emerg Med. 2020 Jul;76(1):115-117.
- ↑ Gawronska K, Lorkowski J. Falls as One of the Atypical Presentations of COVID-19 in Older Population. Geriatric Orthopaedic Surgery & Rehabilitation. January 2021.
- ↑ Bianchetti A, Bellelli G, Guerini F. et al. Improving the care of older patients during the COVID-19 pandemic. Aging Clin Exp Res 2020; 32:1883–1888.
- ↑ Cudjoe TKM, Kotwal AA. "Social Distancing" Amid a Crisis in Social Isolation and Loneliness. J Am Geriatr Soc. 2020 Jun;68(6):E27-E29.
- ↑ 36.0 36.1 36.2 36.3 36.4 36.5 Cox NJ, Morrison L, Ibrahim K, Robinson SM, Sayer AA, Roberts HC. New horizons in appetite and the anorexia of ageing. Age and Ageing 2020; 49(4): 526–534
- ↑ van der Meij BS, Wijnhoven HAH, Lee JS et al. Poor appetite and dietary intake in community-dwelling older adults. J Am Geriatr Soc 2017; 65: 2190–7.
- ↑ Neumann-Podczaska A, Al-Saad SR, Karbowski LM, Chojnicki M, Tobis S, Wieczorowska-Tobis K. COVID 19 - Clinical Picture in the Elderly Population: A Qualitative Systematic Review. Aging Dis. 2020 Jul 23;11(4):988-1008.
- ↑ Molfino A, Kaysen GA, Chertow GM, Doyle J, Delgado C, Dwyer T, Laviano A, Rossi Fanelli F, Johansen KL. Validating Appetite Assessment Tools Among Patients Receiving Hemodialysis. J Ren Nutr. 2016 Mar;26(2):103-10
- ↑ Wang D, Hu B, Hu C, et al. Clinical Characteristics of 138 Hospitalized Patients With 2019 Novel Coronavirus–Infected Pneumonia in Wuhan, China. JAMA. 2020;323(11):1061–1069.
- ↑ 41.0 41.1 41.2 Gaber TA-Z. Assessment and management of post-COVID fatigue. Progress in Neurology and Psychiatry 2021, 2.Wiley Clinical Healthcare Hub (accessed 29 Oct 2021).
- ↑ Townsend L, Dyer AH, Jones K, Dunne J, Mooney A, et al. Persistent fatigue following SARS-CoV-2 infection is common and independent of the severity of initial infection. PLOS ONE 2020;15(11): e0240784.
- ↑ What is Chronic Fatigue Syndrome. Available from: http://www.youtube.com/watch?v=5XEMqDa92RA [last accessed 29/10/2021]
- ↑ What is a Likert Scale. 4 Expert Tips for Creating a Likert Scale. Available from: http://www.youtube.com/watch?v=muYzcuQoyds [last accessed 29/10/2021]