Von Willebrand Disease
Introduction:[edit | edit source]
Von Willebrand Disease (VWD) is considered to be the most common bleeding disorder in humans and in some animals such as dogs. VWD is named after the Finnish physician who described the disease in the 1920s. 1 in 100 to 10 000 individuals are estimated to have VWD. Patients with mild VWD symptoms are rarely diagnosed resulting in the gap “100 to 10 000“[1]. It is estimated that it affects 1% of all US population[2].
History:[edit | edit source]
In 1926 the Finnish physician Erik Von Willebrand described a new bleeding disorder which he called “hereditary pseudo hemophilia.” He recognized that this new disease is different from hemophilia; but he couldn’t identify the responsible plasma factor. Later by many years; this factor was identified and name after him too and now it’s known as Von Willebrand Factor (VWF)[3].
Von Willebrand Factor:[edit | edit source]
Von Willebrand Factor (VWF) is a multifunctional multimeric glycoprotein[4] which is synthesized in endothelial cells[5]. The VWF consists of similar subunits that contains binding sites for glycoprotein receptors. The adhesive activity of VWF depends mainly on the size of its multimers[6] . Von Willebrand Factor plays an important role in primary and secondary hemostasis, acts as a mediator for adhesion and a carrier for coagulation FVIII.[5] It is also involved in angiogenesis and inflammatory processes.[7]
Video 1: Von Willebrand Factor and Coagulation Process
Definition:[edit | edit source]
Von Willebrand Disease can either be inherited or acquired. VWD as an autosomal inherited disease results from a defect in the protein factor called VWF. Type 1 and 2 are autosomal dominant and type 3 is transmitted as autosomal recessive. It affects men and women equally as it is an autosomal linked disease[3][8][9]. Acquired VWD is different from inherited VWD as the disease develops later in life and is not as a result of genetic inheritance. Acquired VWD may be due to autoimmune reactions in people with cardiac defects; certain forms of cancers; diabetes mellitus; autoimmune disease or after usage of certain drugs such as valpronic acid.[10]
Classification of Inherited Von Willebrand Disease:[edit | edit source]
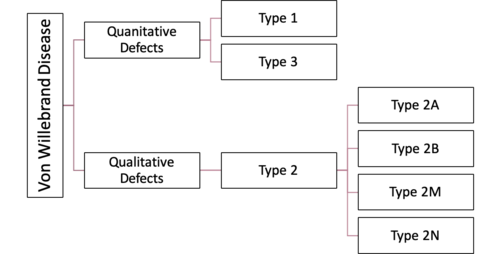
There are several classifications of VWD (Figure 1). There is inherited VWD which is further categorized into type 1, 2 and 3 and acquired VWD. The international society of thrombosis and homeostasis further classifies VWD according to qualitative and quantitative VWF defects[11].
Type 1 Von Willebrand Disease:[edit | edit source]
Type 1 VWD is the most common. About 75% of people with VWD are type 1 [12][13]. It is considered to be the mildest type and it has a partial quantitative VWF defect[12]. Due to its mild presentation it is the hardest to diagnose. Type 1 VWD is autosomal dominant16. The most common manifestation of Type 1 VWD is tooth bleeding (Figure 2) and bleeding post-operative or post injury. [12][14]
Type 2 Von Willebrand Disease:[edit | edit source]
20-25% of people with VWD have type 2, making it the second most common[13]. It is known by its qualitative VWF defect. 15 With Type 2 there is enough VWF factor in the blood. But, the VWF factor does not work properly due to a mutation in VWF multimer[5][15]. It is subdivided into four subtypes which are Type 2A, Type 2B, Type 2M and Type 2N[4].
Type 2A:[edit | edit source]
Type 2A is the most common subtype in Type 2. The amount of VWF is normal but due to a mutational defect in VWF protein, the platelets can’t bind to each other properly. This causes a problem in the coagulation process1[3]. Type 2A manifests by postoperative hemorrhage, menorrhagia and post-dental extraction hemorrhage.[4]
Type 2B:[edit | edit source]
Type 2B is the second most common subtype in Type 2. It has a different mutations in the VWF protein from Type 2A. This defect leads to the binding of the VWF to the platelets in the blood stream instead of binding at the injured site. The body then removes the abnormally bound platelets causing a decrease in the platelets amount[13]. Type 2B manifests by bruising easily, prolonged bleeding from minor wounds and nasal bleeding (epitaxis)[5].
Type 2M:[edit | edit source]
M stands for Multimer. Type 2M is characterized by a mutation in the multimer of the VWF protein leading to decreased activity of VWF and its failure to bind with the platelets. Type 2 M manifests by prolonged bleeding from minor wounds similar to Type 2B[10].
Type 2N:[edit | edit source]
“N “ refers to Normandy or in French Normaundie, a town in France where this subtype was firstly identified.[13] It is characterized by a failure of Factor VIII transporting on VMF despite normal platelet binding with VWF. This results in low factor VIII levels. Type 2N is commonly mistaken for hemophilia A due to the low factor VIII levels.[13]
Type 3 Von Willebrand Disease:[edit | edit source]
The most severe and the rarest type of VMD is type 3 VWD. It is characterized by complete absence of VWF in the blood plasma and blood platelets. In contrast to type 1 and type 2, type 3 is autosomal recessive. Type 3 VWD manifests as severe bleeding in soft tissues, joints, muscle, nose and gut[12].
Clinical Manifestations:[edit | edit source]
The clinical manifestation of VWD is different according to the type of VWD[12].
The most common manifestations includes:[16]
· Nasal hemorrhage.
· Dental and oral cavity hemorrhage.
· Prolonged wound healing.
· Menorrhagia which is excessive menstrual hemorrhage.
· Gastrointestinal hemorrhage (with severe type).
There are some common manifestations in the pediatric population that includes:[17]
· Umbilical stump hemorrhage.
· Cephalic hematoma.
· Cheek hematoma.
· Conjunctival hemorrhage.
· After circumcision hemorrhage.
· After venipuncture hemorrhage.
Diagnostic Tools:[edit | edit source]
Von Willebrand disease is the most common bleeding disorder; but it is also the hardest to diagnos.[18] Unfortunately the usual blood clotting screening laboratory tests such as CBC, Activated Partial Thromboplastin Time (APTT) Test, Prothrombin Time (PT) Test and Fibrinogen test are all normal in patients with VWD especially those with mild and moderate types.[19]
To diagnose VWD there are screening and diagnostic laboratory tests that can be used.[20]
VWD Screening Tests[edit | edit source]
VWF antigen (VWF:Ag):[edit | edit source]
VWF anitigen is a quantitative reliable assessment tool of the plasma VWF protein level24. This method is efficient in detecting VWD quantitative defect types. The normal range of VWF:Ag is 50 to 200IU/dl. Anything lower than 50 may indicate the presence of VWD.[21]
VWF ristocetin cofactor activity (VWF:RCo):[edit | edit source]
The VWF:RCo is the most commonly used test to assess the ability of binding ability of VMF24. The normal ranges of VWF:RCo are between 50 and 200 IU/dL.[21]
Factor VIII activity (FVIII:C):[edit | edit source]
The measurement of FVIII:C is included in the screening laboratory tests of VWD. VWF is a carrier protein for FVIII. Normal ranges of FVIII:C/VWF:Ag ratio is approximately 1. In type 2N, this ratio is low, and in type 3 VWD, the FVIII:C is less than10 IU/dL[21].
VWF:RCo/VWF:Ag Ratio:[edit | edit source]
The VWF:RCo/VWF:Ag ratio is used to diagnose the type of VWD. In Type 1 VWD the levels of both VWF:RCo and VWF:Ag decreases and as such the ratio between them remains around one. While in type 2 VWD the VWF:RCo decreases compared to the VWD:Ag level so the VWF:RCo/VWF:Ag is approximately 0.6[21]
VWD Confirmatory Tests[edit | edit source]
Once VWD is diagnosed some confirmatory tests are run to indicate the type of VWD such as[21]:
· VWF multimer distribution which is found to be abnormal in type 2A and type 2B.
· VWF:CB is abnormal in type 2A and type 2B, some type 2M.
· VWF:PB increases in type 2B.
· LD-RIPA increases in type 2B.
· VWF:FVIIIB decreases in type 2N .
· VWFpp/VWF:Ag ratio increases in type 1.
· VWF gene sequencing is most helpful in differentiating type 2 variants.
Video 2: What is von Willebrand Disease?
Medical Management:[edit | edit source]
Treatment of VWD is based on the severity symptoms and amount of hemorrhage. As most of patients with VWD are type 1 (mild type); they don’t need regular treatment. The goals of treatment is to increase the circulating VWF activity and reduce hemorrhage.[22]
Some medications are used in treating and decreasing the symptoms of VWD such as:
Desmopressin:[edit | edit source]
Desmopressin can be administered by nasal spray, intravenous or subcutaneous injection. It that can be administered easily at home and is used for type 1 VWD. The recommended dosage 0.3 mcg/kg intravenous or subcutaneous or 2 sprays intranasally (for patients above 50 kg) or 1 spray intranasal (for patients less than 50 kg).[23]
Plasma-derived VWF and FVIII Concentrates:[edit | edit source]
Plasma-derived VWF and FVIII concentrate such as Humate P (VWF:RCo: FVIII ratio= 2.4:1), Wilate (VWF:RCo: FVIII ratio = 1:1) and Alphanate Plasma-derived VWF and FVIII concentrate (VWF:RCo: FVIII ratio 1:3) are intravenous medication that are used as an acute treatment in severe types of VWD. They can also act as prophylaxis against hemorrhage. The recommended dosage is 50-60 ristocetin cofactor activity units/kg for major surgery, depending on baseline VWF level and desired goal level.[23]
Antifibrinolytics:[edit | edit source]
Antifibrinolytics such as Aminocaproic acid and Tranexamic acid inhibits fibrinolysis. They are used as active and prophylactic treatments, especially for mucosal surfaces. They are introduced orally or intravenously. The recommended dosage for Aminocaproic acid is 100 mg/kg then 50 mg/kg every 6 hours. The dosage for Tranexemic acid is 1500 mg 3 times daily for 5 days for menorrhagia cases.[22][23]
Hormonal therapy:[edit | edit source]
Hormonal therapy is also an option in treating menorrhagia.[24]
Physical Therapy Management:[edit | edit source]
Physical therapy has an important role in promoting functional skills in pediatrics and adults with VWD; that is why the National Hemophilia Foundation formed a physical therapy working group to create the best physical therapy practice for bleeding disorders such as VWD[25].
The Medical and Scientific Advisory Council (MASAC) developed guidelines and a framework for physical therapy management in bleeding disorders. MASAC stated that physical therapy is crucial in joint and muscles rehabilitation post soft tissue injuries and hemarthroses These clinical presentations mostly occur with the more severe types of VWD.
Physical Therapy Evaluation[edit | edit source]
According to the MASAC; the physical therapy evaluation is an important element of VWD management. The aim of evaluation is to detect musculoskeletal and other limitations caused by the bleeding disorder that affect functional activities and daily life activities(ADL)[26].
History and Interview[26]:[edit | edit source]
It includes interviewing the patient or the caregiver and take the notes about:
- Personal history.
- Family history.
- Bleeding history.
- Medical and surgical history.
- Pain history.
- ADL concerns.
- Occupational concerns.
Joints that have recurrent bleeding disorders are known as “Target Joints”. The most common target joints are knee, elbow, ankle, hip and shoulders.[25]
Subjective Assessment:[edit | edit source]
· Palpation of joints at rest and during active range of motion to detect crepitus, synovitis, edema or temperature.
· Girth measurement to assess edema/ muscle atrophy.
· Atypical Joint End feel detection via passive range of motion.
· Manual Muscle Testing to assess muscular strength.
· Muscle Flexibility test.
· Sensation and proprioception.
Objective Assessment:[edit | edit source]
· Balance and fall assessment.
· Posture and alignment assessment.
· Assessment of functional activities.
· Gait analysis.
· Neuromotor assessment.
· Musculoskeletal Ultrasound.
Physical Therapy Treatment:[edit | edit source]
There is a recommended physical therapy program by the MASAC for muscles and joints bleeding in different recovery phases (Acute, Subacute and Chronic). All muscles follow the same guidelines, except for iliopsoas muscle, and all physical therapy suggested protocols are performed post factor replacement medication as follows :[27]
| Acute Phase | Subacute Phase | Chronic | Other Treatment Considerations | Precautions | |
| Muscles Bleeding except iliopsoas | Main Problem:
Present pain at rest and with movement |
Main Problem :
Limitations in ADL without increasing pain from baseline |
Main Problem :
ADL limitation but without pain |
- Electric stimulation
- Work closely with hematologist - Teach patient to avoid overstretching - Education on activity modification - Kinesiotape® - Treatment duration will vary based on individual needs - Ultrasound for blood absorption can be used with precautions. |
- Monitor for neurovascular compromise while Splinting
- Caution with use of compression on affected muscle. - Use of heat modalities including ultrasound with precautions |
| Physical Therapy Program:
- No compression in case of neuromuscular symptoms. - No active movement or weight bearing till bleeding stoppage. - Splinting. - RICE: Rest, Ice, Compression & Elevation - TENS |
Physical Therapy Program:
- Splinting and assistive device to limit activity - Toe touch Weight bearing - Isometric contractions - Active range of motion - Positioning - AAROM without pain - TENS |
Physical Therapy Program:
- Full Weight Bearing without assistive devices - Positional stretching - Active Range of Motion exercises - unrestricted lifting - Re-evaluation. | |||
| Iliopsoas Muscle Bleeding | Main Problem:
pain presented at rest and with movement |
Main Problem :
ADL limitation without increase pain from baseline |
Main Problem :
ADL limitation but without pain |
- Electrical-stimulation to prevent femoral nerve palsy/atrophy.
- Hip flexor stretching with caution. - Teach patient to avoid overstretching - Treatment duration will vary based on individual |
Monitor for femoral nerve palsy |
| Physical Therapy Program :
- Bedrest - Toe Touch Weight Bearing for household mobility - Rest - Opposite limb ROM Ankle pumps involved lower limb with pain avoidance |
Physical Therapy Program:
- Toes touch weight bearing without increase in pain - Isometric contractions - Active Range Of Motion exercises to involved Lower limb without increase in pain - Positioning with increasing hip extension Range Of Motion in supine and prone over pillows without increase in pain. - therapeutic exercises for non-involved limb |
Physical Therapy Program:
- Full Weight Bearing without assistive devices - positional stretches from prone position - Active range of motion exercises - Re-evaluation | |||
| Joint Bleeding | Main Problem:
pain presented at rest and with movement |
Main Problem :
ADL limitation without increase pain from baseline |
Main Problem :
ADL limitation but without pain |
- TENS
- Relaxation Techniques - Kinesiology tape - Cryotherapy - Elastic stockinette - Myofascial release - Work with hematologist. - Further MRI to determine the presence of chronic synovitis) - Treatment duration will vary based on individual needs. |
- Avoid aggressive exercise too early
- Monitor for nerve compression - Use of heat modalities including ultrasound with precautions - No return to sports or activity until pain-free full ROM and strength |
| Physical Therapy Program :
- RICE (ice for 10-20 minutes every 1-2 hours) - Splinting - Non Weight Bearing using assistive device |
Physical Therapy Program :
- Continue RICE for pain and after exertion Splinting - Night resting splint for protection - Begin progressive weight bearing - Activity modification to avoid pain - Pain-free gentle active Range of motion - Pain-free progressive strengthening |
Physical Therapy Program :
- Dynamic splinting to increase ROM - Active range of motion Progressive - Strengthening as Open chain, closed chain and resistive band - proprioceptive training - Gentle joint mobilizations - Modified functional activities - Orthotics - Reevaluations |
References[edit | edit source]
- ↑ Genetic Home of Reference. Von Willebrand Disease. U.S. National Library of Medicine. Published 2020. Accessed June 15, 2020.
- ↑ Von Willebrand Disease. National Hemophilia Foundation. Published 2020. Accessed June 15, 2020.
- ↑ 3.0 3.1 3.2 Leebeek FWG, Eikenboom JCJ. Von Willebrand’s disease. N Engl J Med. 2016;375(21):2067-2080. doi:10.1056/NEJMra1601561
- ↑ 4.0 4.1 4.2 Rassoulzadegan M, Ala F, Jazebi M, et al. Molecular and clinical profile of type 2 von Willebrand disease in Iran: a thirteen-year experience. Int J Hematol. 2020;111(4):535-543. doi:10.1007/s12185-019-02814-8
- ↑ 5.0 5.1 5.2 5.3 Randi AM, Laffan MA. Von Willebrand factor and angiogenesis: basic and applied issues. J Thromb Haemost. 2017;15(1):13-20. doi:10.1111/jth.13551
- ↑ Stockschlaeder M, Schneppenheim R, Budde U. Update on von Willebrand factor multimers: focus on high-molecular-weight multimers and their role in hemostasis. Blood Coagul Fibrinolysis. 2014;25(3):206-216. doi:10.1097/MBC.0000000000000065
- ↑ Lenting PJ, Casari C, Christophe OD, Denis C V. von Willebrand factor: the old, the new and the unknown. J Thromb Haemost. 2012;10(12):2428-2437. doi:10.1111/jth.12008
- ↑ Sharma R, Haberichter SL. New advances in the diagnosis of von Willebrand disease. Hematology. 2019;2019(1):596-600. doi:10.1182/hematology.2019000064
- ↑ Echahdi H, El Hasbaoui B, El Khorassani M, Agadr A, Khattab M. Von Willebrand’s disease: case report and review of literature. Pan Afr Med J. 2017;27:147. doi:10.11604/pamj.2017.27.147.12248
- ↑ 10.0 10.1 Nazzaro A-M, Philipp CS, Johnson RW, James AH. Von Willebrand Disease. National Organization of Rare Diseases.. Published 2020. Accessed June 15, 2020.
- ↑ Bharati KP, Prashanth UR. Von Willebrand disease: an overview. Indian J Pharm Sci. 2011;73(1):7-16. doi:10.4103/0250-474X.89751
- ↑ 12.0 12.1 12.2 12.3 12.4 Von Willebrand Disease. NHS. https://www.nhs.uk/conditions/von-willebrand-disease/. Published 2017. Accessed June 16, 2020.
- ↑ 13.0 13.1 13.2 13.3 13.4 Types of von Willebrand disease. Canadian Hemophilia Society. https://www.hemophilia.ca/types-of-von-willebrand-disease/. Published 2018. Accessed June 16, 2020.
- ↑ Vanduine S, Ridley K, Bashutski J, Snyder M, Powell C, Taichman S. Gingival bleeding and oral hygiene in women with von Willebrand Disease (VWD): a pilot study. J Haemoph Pract. 2018;4(1):49-57. doi:10.17225/jhp00096
- ↑ Hemophilia of Georgia. Types of von Willebrand Disease. The Hemophilia, von Willebrand Disease & Platelet Disorders Handbook. Published 2007. Accessed June 16, 2020.
- ↑ Pollak ES. Von Willebrand Disease Clinical Presentation. Medscape. https://emedicine.medscape.com/article/206996-clinical. Published 2019. Accessed June 16, 2020
- ↑ Sanders Y V, Fijnvandraat K, Boender J, et al. Bleeding spectrum in children with moderate or severe von Willebrand disease: Relevance of pediatric-specific bleeding. Am J Hematol. 2015;90(12):1142-1148. doi:10.1002/ajh.24195
- ↑ Castaman G, Linari S. Diagnosis and Treatment of von Willebrand Disease and Rare Bleeding Disorders. J Clin Med. 2017;6(4):45. doi:10.3390/jcm6040045
- ↑ National Center on Birth Defects and Developmental Disabilities. von Willebrand Disease (VWD) Diagnosis. Centers for Disease Control and Prevention. https://www.cdc.gov/ncbddd/vwd/diagnosis.html. Published 2019. Accessed June 17, 2020.
- ↑ Roberts JC, Flood VH. Laboratory diagnosis of von Willebrand disease. Int J Lab Hematol. 2015;37(1):139-148. doi:doi:10.1111/ijlh.12345
- ↑ 21.0 21.1 21.2 21.3 21.4 Ng C, Motto DG, Paola J Di. Diagnostic approach to von Willebrand disease. Blood. 2015;125(13):2029-2037. doi:10.1182/blood-2014-08-528398
- ↑ 22.0 22.1 Chapin J. Von Willebrand disease in the elderly: clinical perspectives. Clin Interv Aging. 2018;13:1531-1541. doi:10.2147/CIA.S136931
- ↑ 23.0 23.1 23.2 Sharma R, Flood VH. Advances in the diagnosis and treatment of Von Willebrand disease. Hematology. 2017 Dec 8;2017(1):379-84.
- ↑ Green D. Diagnosis and Treatment of von Willebrand Disease. NEJM Jornal Watch. https://www.jwatch.org/na45666/2017/12/06/diagnosis-and-treatment-von-willebrand-disease. Published 2017. Accessed June 17, 2020.
- ↑ 25.0 25.1 Lowes LP, Alfano L, Orlin MN. Muscloskeltal System: Considerations and Interventions for Specific Pediatric Pathology. In: Effgen SK, ed. Meeting the Physical Therapy Needs of Children. 2nd ed. Philadelphia: F. A. Davis Company; 2013:226-227
- ↑ 26.0 26.1 Medical and Scientific Advisory Counsil. Physical Therapy Evaulation Recommendations. Natl Hemoph Found. 2018
- ↑ National Hemophilia Foundation. Physical Therapy Practice Guidelines for Persons with Bleeding Disorders: Muscle Bleed. 2018;