Traumatic Experiences: Vagus Nerve, Microbiome, Heart Rate Variability (HRV) and Effects of Exercise
Original Editor - Andrea Sturm
Top Contributors - Vidya Acharya, Kim Jackson, Jess Bell, Tarina van der Stockt and Lucinda hampton
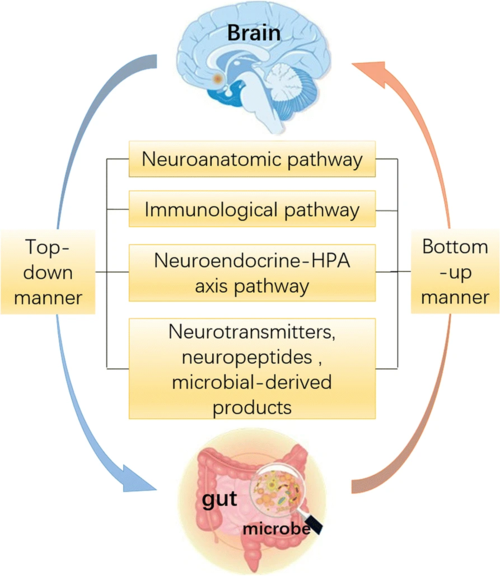
The Brain-Gut-Microbiome Axis[edit | edit source]
Biological psychiatry research has long focused on the brain in elucidating the neurobiological mechanisms of anxiety- and trauma-related disorders. A review challenges this assumption and suggests that the gut microbiome and its interactome also deserve attention to understand brain disorders and develop innovative treatments and diagnostics in the 21st century. The recent, in-depth characterization of the human microbiome spurred a paradigm shift in human health and disease. Animal models strongly suggest a role for the gut microbiome in anxiety- and trauma-related disorders. The microbiota–gut–brain (MGB) axis sits at the epicenter of this new approach to mental health. The microbiome plays an important role in the programming of the hypothalamic–pituitary–adrenal (HPA) axis early in life, and stress reactivity over the life span.[1]
Preclinical and clinical studies have shown bidirectional interactions within the brain-gut-microbiome axis. Gut microbes communicate to the central nervous system through at least 3 parallel and interacting channels involving nervous, endocrine, and immune signalling mechanisms. The brain can affect the community structure and function of the gut microbiota through the autonomic nervous system, by modulating regional gut motility, intestinal transit and secretion, and gut permeability, and potentially through the luminal secretion of hormones that directly modulate microbial gene expression.[2]
A system biological model is proposed that posits circular communication loops amid the brain, gut, and gut microbiome, and in which perturbation at any level can propagate dysregulation throughout the circuit. A series of largely preclinical observations implicate alterations in brain-gut-microbiome communication in the pathogenesis and pathophysiology of irritable bowel syndrome, obesity, and several psychiatric and neurologic disorders. Continued research holds the promise of identifying novel therapeutic targets and developing treatment strategies to address some of the most debilitating, costly, and poorly understood diseases.[2]
The Microbiome in Posttraumatic Stress Disorder[edit | edit source]
Recent research has focused on the role of exaggerated inflammatory responses in the pathogenesis of Post Traumatic Stress Disorder (PTSD.) To this end, a subset of CD4+ T cells, the regulatory T cells (Tregs), have been found to be altered in PTSD-affected individuals. Tregs play an important role in defence against inappropriate inflammatory responses, such as those observed in autoimmunity, allergy, and asthma. Reduced levels of Treg cells have been observed following exposure of human participants to a laboratory stressor, and in male and female refugees with chronic PTSD, relative to healthy controls. Furthermore, reduced frequency of Tregs is associated with autoimmune diseases such as thyroiditis, inflammatory bowel disease, and rheumatoid arthritis, conditions for which individuals with PTSD show increased risk[5]
Consistent with these findings, genome-wide association studies in PTSD cohorts revealed association with ANKRD55, a gene associated with several autoimmune and inflammatory disorders, including multiple sclerosis, type 2 diabetes mellitus, celiac disease, and rheumatoid arthritis.[5] As a sideline - the association between childhood trauma and multiple sclerosis (MS) has been investigated by comparing histories of child abuse and neglect between patients with MS and adults from the general population in a cross-sectional case-control study. The self-reported Childhood Trauma Questionnaire for the assessment of emotional, physical, and sexual abuse and emotional and physical neglect was administered to 234 patients with definite MS and 885 adults from the general population.[6]
After adjusting for sociodemographic factors and current depression, patients with MS scored significantly higher in all Childhood Trauma Questionnaire subscales apart from physical abuse and neglect than adults from the general population. Adjusted odds ratios for these types of childhood trauma were higher in the MS group than in controls, ranging from 2.0 for emotional neglect (95% confidence interval = 1.3-3.2) to 3.4 for emotional abuse (95% confidence interval = 2.0-5.7). Although childhood trauma was not associated with the degree of current MS-related disability, patients with MS with histories of physical and/or sexual abuse had significantly higher relapse rates than patients without early-life stress.[6]
Jergovic et al. observed an altered Treg phenotype in male combat veterans with PTSD compared to healthy controls. PTSD has also been found to result in upregulation of interleukin (IL) 6 and proinflammatory cytokines, including interferon gamma (IFN-γ), IL-1β and tumor necrosis factor (TNF). Elevated levels of C-reactive protein (CRP), a clinically used marker of inflammation, have also been observed in individuals with PTSD. Pre-existing elevated CRP levels, or elevated IL-6 measured within 24 hours following trauma, have been found to predict post-deployment CAPS scores in war zone-deployed Marines or a diagnosis of PTSD in children six months following trauma, respectively.[5]
An important factor determining immunoregulation, indicated by a balanced expansion of effector T cell populations and Tregs, is the human microbiome. The human microbiota comprises all the microorganisms (archaea, bacteria, eukaryotes, fungi, and viruses) harbored by the human body and the complete catalog of these microbial symbionts and their genes constitute the human microbiome. Research suggests that microbial inputs are essential for maintaining homeostasis and optimum health, controlling blood-brain barrier permeability, and regulating central nervous system (CNS) function. A complex, bidirectional system of communication exists between the gut microbiome, the gut, and the CNS.[5]
Data from animal studies indicate that environmental and gut microbial species elicit a significant impact on cognitive function, memory, and fundamental patterns of behaviour, such as social interaction and stress coping. Additionally, stress can influence the composition of the gut microbiota, and the bidirectional communication between microbiota and the CNS, in turn, influences stress reactivity. Alterations in microbiota have been shown to modulate plasticity-related, serotonergic, and GABAergic signalling systems in the CNS. Dysregulation of the gut microbiome (dysbiosis) therefore may influence risk of developing a disease, including stress- or trauma-related disorders.[5]
Exploring the gut microbiota in inpatients with bipolar disorder during a depressive episode[edit | edit source]
Bipolar affective disorder (BD) is one of the top 10 causes of global disability and premature mortality with enormous socioeconomic impact. Nevertheless, the neurobiological basis of BD is not sufficiently characterized. Relevant factors include structural brain changes, disturbances in neuroplasticity as well as chronobiology. Pathophysiological causes are genetic and environmental factors, including defects in apoptotic, immune‐inflammatory, neurotransmitter, neurotrophin and calcium signalling pathways. Additionally, alterations in oxidative and nitrosative stress, cellular bioenergetics, and membrane or vesicular transport have been found in BD.[7]
Gut microbiota research in BD is still in its infancy, and the communication pathways between the microbiota and the brain have not yet been identified in detail. Because of the dynamic and changing microbial community, which is highly modifiable by external lifestyle parameters such as diet and physical activity, any mechanistic relationships are difficult to study.[7] Although the relationship between diet, the gut microbiota, host immunity and host metabolism is becoming more evident, the relationship between the microbiota and exercise has not been fully explored. Study findings showed that a combination of exercise and diet impacts on gut microbial diversity. In particular, the enhanced diversity of the microbiota correlated with exercise and dietary protein consumption in an investigated athlete group. Diversity is important in all ecosystems to promote stability and performance.[8]
From a psychiatric perspective, it is interesting that the association between neurotransmitters and gut microbiota has already been investigated. The results showed that most of our neurotransmitters can be produced by gut bacteria. For example, Lactobacillus species produce acetylcholine and GABA; Bifidobacterium species also synthesize GABA; Escherichia produces noradrenaline, 5‐HT and dopamine; Streptococcus and Enterococcus synthesize 5‐HT, and Bacillus species produce dopamine and noradrenaline. TRP, which is a 5‐HT precursor, has been shown to be altered in BD along with changes in KYN pathway metabolites. Psychopharmacological agents such as antidepressants and lithium have been shown to have antibiotic properties, which could affect the gut microbiota in the short and long term. As BD is highly comorbid with inflammatory diseases and obesity, it is conceivable that complex interactions between the investigated parameters are responsible for the detected changes of the gut microbiota.[7]
We can conclude that metabolic syndrome and low-grade inflammation are important trademarks for BD that could be possibly affected by the gut microbiota. Study results point out that BD may not uniquely be an affective disorder secondary to cerebral monoamine dysregulations but could also be understood in the wider context of gut-brain axis dysfunction.[7]
Exposure to potentially traumatic events (PTEs) is known to increase the risk for a mental disorder. Cumulative exposure to PTEs in conflict-affected communities, including mass violence and human-rights abuses, is a strong predictor of PTSD, depression and anger/Intermittent Explosive Disorder (IED) - an association that is known as the dose-effect relationship. Longitudinal studies amongst veteran and other trauma-exposed populations have also shown that posttraumatic stress symptoms following PTE exposure may precede and contribute to the onset of depression and other anxiety disorders over time. Liddell, Kemp, Steel et al. investigated diminished heart rate variability (HRV) – an index of vagus nerve function and a robust predictor of emotion regulation capacity - as a vulnerability marker that potentially mediates the association between PTE exposure, age and symptoms of posttraumatic stress disorder (PTSD), psychological distress and aggressive behavior. [9]
Trauma, Heart Rate Variability (HRV) and the Vagus Nerve[edit | edit source]
One potential psychophysiological marker of increased sensitivity to a range of affective responses following trauma is reduced heart rate variability (HRV), reflecting altered vagal nerve function and impaired emotion regulation capacity. HRV indexes parasympathetic regulation of heart rate via the inhibitory influence of the myelinated vagus nerve over the sinoatrial node. As such, under conditions of safety, the heart rate is slowed, and social engagement is facilitated. In response to a salient external cue – such as a threat signal – the vagal brake is released, allowing the sympathetic nervous system to dominate and mobilize defence responses. Exposure to chronic threat in a conflict-affected context may affect the functioning of the vagal system in the long term, impairing adaptive reactions to stressful events[9].
A higher level of resting-state HRV has been postulated to reflect a central indicator of healthy emotion regulation capacity, indicating a system that is able to effectively respond to environmental challenges. Accordingly, a healthy vagal system is thought to index psychological flexibility, emotional self-regulation and positive adaptation. Conversely, low resting state HRV is an indicator of psychophysiological rigidity characterized by a diminished capacity for regulating emotional responses to distressing events. Reduced resting HRV has been associated with greater trauma exposure, mental disorder and symptoms, and physical health status, including PTSD; depression and anxiety; aggression and anger; comorbidity, and poor physical health. Studies have also linked reduced resting HRV with increased vulnerability to distress and delayed physiological recovery following stress exposure.[9]
Improvements in heart rate variability with exercise therapy[edit | edit source]
Heart rate variability (HRV) is a noninvasive, practical, and reproducible measure of autonomic nervous system function. A heart rate that is variable and responsive to demands is believed to bestow a survival advantage, whereas reduced HRV may be associated with poorer cardiovascular health and outcomes. In recent years, many researchers have investigated the prognostic implications of HRV in a variety of clinical populations. Evidence suggests that reduced HRV has prognostic significance for individuals with myocardial infarction, chronic heart failure, unstable angina, and diabetes mellitus. Interventions to increase HRV, such as exercise therapy, have also been examined. The findings suggest that exercise therapy may improve HRV in myocardial infarction, chronic heart failure, and revascularization patients by increasing vagal tone and decreasing sympathetic activity. One hypothesis is that a shift toward greater vagal modulation may positively affect the prognosis of these individuals. While the underlying mechanisms by which exercise training improves vagal modulation are speculative at present, angiotensin II and nitric oxide may be potential mediators.[11]
Potential mechanism in the modification of HRV by exercise therapy[edit | edit source]
Enhanced cardiac vagal tone may offer a survival advantage. Greater vagal influence decreases the amount of work and oxygen consumed by the heart via a reduction in resting heart rate and myocardial contractility. It appears that stimulation of the vagus nerve directly acts on the sinus node and the myocardium, and hinders sympathetic influences. Cardiac vagal tone may also reduce the risk of frequently lethal ventricular dysrhythmias including ventricular fibrillation.[11]
Exercise training may enhance vagal tone. While the underlying mechanisms by which exercise training improves vagal modulation are speculative at present, angiotensin II and nitric oxide (NO) are potential mediators. A potential mechanism underlying the exercise training-cardiac vagal tone association is angiotensin II. Angiotensin II is known to inhibit cardiac vagal activity. One theory is that exercise training suppresses angiotensin II expression. Researchers have also discovered that plasma renin activity levels are lower in athletes (long-distance runners) than in untrained individuals or nonathletes and sedentary individuals. This finding is important given that athletes with lower plasma renin activity would presumably have lower angiotensin II and higher associated levels of cardiac vagal activity. Therefore, it is possible that the suppression of angiotensin II via exercise may, to some extent, mediate the enhancement of cardiac vagal tone.[11]
NO may also play a role in increasing cardiac vagal control and, in doing so, may indirectly inhibit sympathetic influences. Exercise training has been found to improve endothelial function and NO bioavailability among individuals with coronary risk or coronary atherosclerosis. Therefore, it is possible that the relationship between exercise and cardiac vagal activity is mediated, at least in part, by NO. However, more research is needed to clarify the possible role of NO in autonomic control as well as its potential influence on the exercise-cardiac vagal tone relationship.[11]
Limited research to date suggests that exercise training (endurance training in particular) increases cardiac vagal tone and reduces sympathetic cardiac influences. Improvements in HRV have been reported in studies of Myocardial Infarct (MI) patients participating in unsupervised low-intensity walking programs as well as more intensive supervised exercise programs. Researchers have also reported that a two-week exercise program consisting of two 30 min daily sessions of cycle ergometry at the anaerobic threshold facilitated parasympathetic nervous system activity recovery after an MI more quickly than for those participating in a walking cardiac rehabilitation program. The exact mechanisms underlying the modification of HRV by exercise therapy are not known.[11]
The autonomic nervous system is highly adaptable and allows the organism to maintain its balance when experiencing strain or stress. Conversely, a lack of flexibility and a rigid system can lead to somatic and psychological pathologies. Several studies have shown a link between reduced HRV in post-myocardial infarction patients and increased risk for adverse cardiovascular events, including ventricular arrhythmias and sudden death. Recently, studies indicate that patients with depression and anxiety disorders exhibit abnormally low HRV compared with non-psychiatric controls. Reduced HRV seems to indicate the decreased cardiac vagal tone and elevated sympathetic activity in anxious and depressive patients and would reflect a deficit in the flexibility of emotional physiological mechanisms.[12]
References[edit | edit source]
- ↑ Martin CR, Osadchiy V, Kalani A, Mayer EA. The brain-gut-microbiome axis. Cellular and molecular gastroenterology and hepatology. 2018 Jan 1;6(2):133-48.
- ↑ 2.0 2.1 Malan-Muller S, Valles-Colomer M, Raes J, Lowry CA, Seedat S, Hemmings SM. The gut microbiome and mental health: implications for anxiety-and trauma-related disorders. Omics: a journal of integrative biology. 2018 Feb 1;22(2):90-107.
- ↑ DavidPerlmutterMD Gut Bacteria Change Your Brain Available from https://www.youtube.com/watch?v=RXh4u03lM3w Accessed on 28/09/19
- ↑ Zhao L, Xiong Q, Stary CM, Mahgoub OK, Ye Y, Gu L, Xiong X, Zhu S. Bidirectional gut-brain-microbiota axis as a potential link between inflammatory bowel disease and ischemic stroke. Journal of neuroinflammation. 2018 Dec;15(1):339.
- ↑ 5.0 5.1 5.2 5.3 5.4 Hemmings SM, Malan-Muller S, van den Heuvel LL, Demmitt BA, Stanislawski MA, Smith DG, Bohr AD, Stamper CE, Hyde ER, Morton JT, Marotz CA. The microbiome in posttraumatic stress disorder and trauma-exposed controls: an exploratory study. Psychosomatic medicine. 2017 Oct;79(8):936.
- ↑ 6.0 6.1 Spitzer C, Bouchain M, Winkler LY, Wingenfeld K, Gold SM, Grabe HJ, Barnow S, Otte C, Heesen C. Childhood trauma in multiple sclerosis: a case-control study. Psychosomatic medicine. 2012 Apr 1;74(3):312-8.
- ↑ 7.0 7.1 7.2 7.3 Painold A, Mörkl S, Kashofer K, Halwachs B, Dalkner N, Bengesser S, Birner A, Fellendorf F, Platzer M, Queissner R, Schütze G. A step ahead: Exploring the gut microbiota in inpatients with bipolar disorder during a depressive episode. Bipolar disorders. 2019 Feb;21(1):40-9.
- ↑ Clarke SF, Murphy EF, O'Sullivan O, Lucey AJ, Humphreys M, Hogan A, Hayes P, O'Reilly M, Jeffery IB, Wood-Martin R, Kerins DM. Exercise and associated dietary extremes impact on gut microbial diversity. Gut. 2014 Dec 1;63(12):1913-20.
- ↑ 9.0 9.1 9.2 Liddell BJ, Kemp AH, Steel Z, Nickerson A, Bryant RA, Tam N, Tay AK, Silove D. Heart rate variability and the relationship between trauma exposure age, and psychopathology in a post-conflict setting. BMC psychiatry. 2016 Dec;16(1):133.
- ↑ Owen Epstein Heart rate variability Available from https://www.youtube.com/watch?v=B6Id6Jl7_HU Accessed on 28/09/19
- ↑ 11.0 11.1 11.2 11.3 11.4 Routledge FS, Campbell TS, McFetridge-Durdle JA, Bacon SL. Improvements in heart rate variability with exercise therapy. Canadian Journal of Cardiology. 2010 Jun 1;26(6):303-12.
- ↑ Servant D, Logier R, Mouster Y, Goudemand M. Heart rate variability. Applications in psychiatry. L'Encephale. 2009 Oct;35(5):423-8.






