Rheumatoid Arthritis
Original Editors - Florence Brachotte Amanda Fetz & Katie Robertson from Bellarmine University's Pathophysiology of Complex Patient Problems project
Top Contributors - Amanda Fetz, Riley Sturzebecher, Kim Jackson, Lucinda hampton, Amanda Ager, Admin, Elaine Lonnemann, Aminat Abolade, Wendy Walker, 127.0.0.1, Katie Robertson, WikiSysop, Simisola Ajeyalemi, Shaimaa Eldib and Amrita Patro Amanda Fetz,Riley Sturzebecher,Elaine Lonnemann,Rachael Lowe
Introduction[edit | edit source]
- Rheumatoid arthritis (RA) is a systemic autoimmune disease characterized by inflammatory arthritis and extra-articular involvement. RA with symptom duration of fewer than six months is defined as early, and when the symptoms have been present for more than months, it is defined as established.
- There is no laboratory test that is pathognomonic for rheumatoid arthritis. The treatment of patients with rheumatoid arthritis requries both pharmacological and non-pharmacological agents. Today, the standard of care is early treatment with disease modifying anti-rheumatic drugs[1]
- RA is a highly disabling disease associated with high morbidity. RA results in considerable direct costs eg health care expenses, and indirect costs, eg. loss of productivity due to morbidity and decreased life expectancy.[2] The increased mortality mainly associated with cardiovascular disease and accelerated atherosclerosis. Atherosclerotic disease is driven by inflammatory mechanisms similar to those in RA. Cardiovascular morbidity correlates with inflammatory activity in RA.
Clinically Relevant Anatomy[edit | edit source]
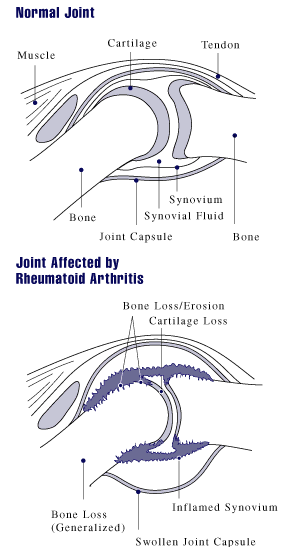
Synovium
- The dense connective-tissue membrane that secretes synovial fluid
- Lines the ligamentous surfaces of joint capsules, tendon sheaths where free movement is necessary, and bursae.
- Normally the synovium consists of 2 - 3 layers of cells.
- In patients with rheumatoid arthritis, the synovium is strongly thickened and inflamed.
- The cause of this inflammation in rheumatoid arthritis is unknown.[3]
The synovium in rheumatoid arthritis is infiltrated by immune cells which include innate immune cells (monocytes, dendritic cells, mast cells) and adaptive immune cells, B cells and plasma cells. Cytokines and chemokines stimulate factors which activate endothelial cells and attract immune cells within the synovial compartment. Fibroblast and inflammatory cells lead to osteoclast generation resulting in bone erosion the hallmark feature of rheumatoid arthritis, and loss of joint integrity what frequently leads to disability. [4] [1]
During the early phase of the disease there is an influx of inflammatory cells into the synovial membrane. As the disease progresses, there is a proliferation of monocytes and thickening of the synovial membrane with small villous projections into the joint space.[1]
Epidemiology[edit | edit source]
RA is found all around the world, but does tend to be more prevalent in the Native American and white population.
Incidence
- 29 cases/100,000 in northern Europe,
- 38/100,000 in North America
- 16.5/100,000 in southern Europe. [1]
Prevelance
- In North America and northern Europe, RA affects 0.4% to 1% of the population
- In southern Europe, it affects 0.3% to 0.7% of the population.
- Female to male ratio is 2-3:1
- Increases with age. RA most commonly begins in :women between the ages of 30 and 60; later in life for men; in the pediatric population usually begins before the age of 16, known as Juvenile Idiopathic Arthritis (JIA)[5][6]
Etiology[edit | edit source]
The cause of Rheumatoid Arthritis remains unknown and can therefore not be prevented. A simple disorganisation of the immune system can be at the origin of the body attacking its own tissue. The evolution of the disease varies from person to person; sometimes the inflammation can become systemic, what means that it will expand and also affect multiple organs, systems or tissues. [3]
Systemic inflammation and autoimmunity in RA begin long before the onset of detectable joint inflammation[7].
- Emerging data suggest that RA-related autoimmunity may be initiated at a mucosal site years before the onset of joint symptoms.
- The candidate sites of origin include the oral, lung and gastrointestinal mucosa, as data consistent with this hypothesis have been generated for each location.eg Changes in the composition and function of intestinal microbiome have been related to rheumatoid arthritis. The composition of gut microbiome is altered in patients with rheumatoid arthritis (dysbiosis), rheumatoid arthritis patients have decreased gut microbiome diversity when compared with healthy individuals. Studies have linked inflammation in the oral cavity and specifically periodontitis to the preclinical period of RA.[8].
- Individual patients may undergo initiation events at unique sites but still converge on similar joint findings as the disease process evolves.
- RA is typically divided into two subtypes designated “seropositive” and “seronegative” disease, with seropositivity being defined as the presence of serum elevations of the autoantibodies rheumatoid factor (RF) and the more recently described antibodies to citrullinated protein/peptide antigens (ACPAs).
- Multiple genetic and environmental factors[8] have been associated with an increased risk for rheumatoid arthritis (RA).
- Cigarette smoking is the strongest environmental risk factor associated with rheumatoid arthritis.[1]
Genetic and Familial Risk Factors for RA[edit | edit source]
There is a generally increased prevalence of RA within families resulting from the interaction between patients genotype and environment.
- Twin studies have shown a concordance rate of 15% to 30% among monozygotic twins and 5% among dizygotic twins. The heritability of rheumatoid arthritis is approximately 40% to 65% for seropositive rheumatoid arthritis and 20% for seronegative rheumatoid arthritis[1].
- Genetic factors in RA are suggested by an increased prevalence of disease within certain racial groups such as North American natives, who exhibit prevalence rates of RA of 5–7%[8]. Although there may be non-genetic familial or cohort factors that play a role in the family or racial/ethnic group risk, multiple specific genetic loci have been identified that are associated with increased risk for RA and in some cases decreased risk.
- The strongest of the genetic risk factors is a set of alleles within the major histocompatibility complex (MHC) that encode amino acid sequences that predict structural similarities in the human leukocyte antigen (HLA) peptide-binding groove and are termed in the aggregate “shared epitope,”(SE). SE alleles are believed to contribute up to ∼40% of the genetic risk for RA, although other studies suggest less contribution[8].
Environmental Factors[edit | edit source]
Multiple environmental, dietary, and lifestyle factors have been associated with RA[8].
- Smoking/Tobacco Exposure: Many studies have found that exposure to smoking accounts for ∼20–30% of the environmental risk for RA. Primarily, smoking is most strongly associated with antibodies to citrullinated protein/peptide antigens (ACPA) - positive RA. It has been proposed that smoking may lead to increased citrullination and in presence of the right genetic background, it may lead to the presentation of citrullinated proteins and the generation of ACPA, along with other local and systemic effects of smoking tobacco influencing the immunity. Smoking has long been associated with the presence of RF even in the absence of RA. This suggests that there may be biologic interactions between these factors that drive the development of RA or at the very least RA-related autoimmunity. Thus, a major unanswered question regarding the role of smoking in RA is where it acts in the natural history of RA. Specifically, does exposure to tobacco smoke act to trigger the initial autoimmunity or does it drive the propagation of autoimmunity to the point of classifiable disease? These issues need to be investigated thoroughly, especially given the potential for smoking to be a modifiable risk factor and therefore a potential preventive intervention in RA development.
- Dietary factors: Lower intake of vitamin D and antioxidants and higher intake of sugar, sodium, red meats, protein, iron and certain medications are associated with increased risk of RA.
| Environmental and other factors associated with rheumatoid arthritis risk[8]. |
|---|
- Increases risk: Female sex; exposure to tobacco; occupational dust (silica); air pollution; high sodium red and iron consumption; obesity; low vitamin D intake and levels.
- Deceased risk: fish and omega 3 fatty acid consumption; moderate alcohol intake; healthy diet; oral contraceptive/HRT; statin use.
Mucosal Processes Influencing RA Development[edit | edit source]
The initial inflammation and autoimmunity in RA begins outside of the joints[7]. RA-related autoimmunity may originate at a mucosal site. The general model[8] underlying a hypothesis that mucosal surfaces (and potentially microbes) play a role in the pathogenesis of RA is as follows.
- At some point in preclinical RA, at a mucosal surface (e.g., the oral cavity, lung, gut) interactions between microbes potentially other environmental factors (e.g., tobacco smoke) and host factors lead to mucosal inflammation and initial breaks in RA-related immune tolerance.
- This mucosal inflammation then facilitates local, and then systemic, propagation of autoimmunity through mechanisms that include molecular mimicry or facilitation of development of direct autoimmunity to self-antigens.
Gender and Rheumatoid Arthritis[edit | edit source]
Female-specific factors influence risk for RA. (many controversies still exist[9]).
- The post-menopause stage, an early age at menopause, the post-partum period and the use of anti-oestrogen agents are associated with RA onset.
- All these phenomena have in common an acute decline in ovarian function and/or in oestrogen bioavailability.
- There are controversies regarding other female hormonal factors.
- The influence of systemic hormonal treatments, including contraceptive and HRT, on RA onset, remains unclear.
- The effect of other factors related to diverse hormonal changes (such as parity, breastfeeding or PCO) is also controversial.
- The timing of oestrogen exposure plays a role in RA onset, female hormonal factors having varying effects during premenopause and post-menopause.
- The effect of sex hormones on the immune system and their interaction with environmental and genetic factors could explain the higher prevalence of RA in women.
- Some female hormonal factors are potentially modifiable, understanding their precise role is key for future preventive interventions focusing on women at high risk[8].
Characteristics/Clinical Presentation[edit | edit source]
In rheumatoid arthritis, joint complaints are in the foreground. Most common clinical presentation of RA is
- Polyarthritis of small joints of hands: proximal interphalangeal (PIP), metacarpophalangeal (MCP) joints and wrist. Some patients may present with monoarticular joint involvement.
- Commonly joint involvement occurs insidiously over a period of months, however, in some cases, joint involvement may occur over weeks or overnight.
- Other commonly affected joints include wrist, elbows, shoulders, hips, knees, ankles and metatarsophalangeal (MTP) joints.
- Stiffness in the joints in the morning may last up to several hours, usually greater than an hour. The patient may have a "trigger finger" due to flexor tenosynovitis.
On examination,
- May be swelling, stiffness, deformity, and tenderness of the PIP, MCP wrist, knee joints, referred to as synovitis, and there may be a decreased range of motion.
- Deformity, pain, weakness and restricted mobility resulting in loss of function.[10]
- Rheumatoid nodules may be present in 20% of patients with rheumatoid arthritis; these occur over extensor surfaces at elbows, heals, and toes.
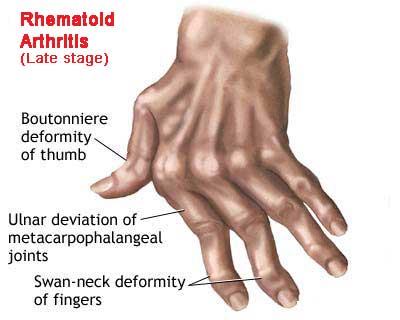
- Late in the course of the disease patient may present with "boutonniere (flexion at PIP and extension at DIP), swan neck (flexion at DIP and extension at PIP) deformities, subluxation of MCP joints and ulnar deviation.
- Other features may include the presence of carpal tunnel syndrome, tenosynovitis and finger deformities.
- Examine the joints on swelling, pain due to palpation, pain due to movement, decreased range of motion, deformation and instability.
- Hallmark symptoms such as symmetrical joint swelling and tenderness, morning stiffness, positive rheumatoid factor (RF), elevated acute phase reactants, and radiographic evidence of erosive bone loss.
- Significant predictors of functional decline among persons with RA are slow gait and a weak grip. [3][11]
Rheumatoid arthritis can affect almost every organ in the body
- The three most important complaints are the pain, morning stiffness and fatigue.
- Muscular strength, muscular endurance and aerobic endurance are typically reduced in patients with rheumatoid arthritis in comparison with healthy patients.
- In 80-90% of the patients with rheumatoid arthritis the cervical spine is involved, which can lead to instability, caused by the ligamentous laxity (between the first and second cervical vertebrae most commonly) This instability can lead to pain and neurological symptoms,eg headache and tingling in the fingers. [3]
- Individuals with RA are 8 times more likely to have the functional disability compared with adults in the general population from the same community.
Signs and Symptoms of RA[edit | edit source]
- Joint Pain: warmth, redness, tenderness, swelling
- Joint Stiffness: increased in the mornings
- Fatigue throughout the body
- Fever
- Weight Loss
- Rheumatoid Nodules: small lumps of tissue felt under the skin
- Symmetrical Patterns of affected joints
- Most common joints: wrist, hand, fingers, cervical spine, shoulder, elbow, knee, hip, foot, and ankle
- Prolong symptoms
- Anemia
- Neck Pain
- Dry Eyes
- Dry Mouth
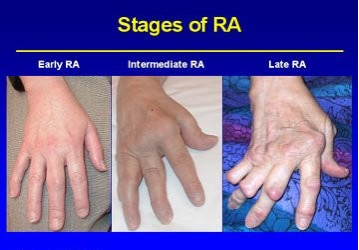
Staging[edit | edit source]
Disease progression:
Stage 1: No destructive changes on x-rays
Stage 2: Presence of x-ray evidence of periarticular osteoporosis, subchondral bone destruction but no joint deformity
Stage 3: X-ray evidence of cartilage and bone destruction in addition to joint deformity and periarticular osteoporosis.
Stage 4: Presence of bony or fibrous ankylosis along with stage 3 features.[1]
Differential Diagnosis[edit | edit source]
- Lupus
- Chronic Lyme disease
- Osteoarthritis
- Septic arthritis
- Psoriatic arthritis
- Sjogren syndrome
- Sarcoidosis[1]
Complications[edit | edit source]
RA has many effects on individuals including mortality, hospitalization, work disability, increase in medical cost/expenses, decreases of quality of life, and chronic pain. On average, the chronic RA patient has two or more comorbid conditions.[12] This is significant because of the comorbidities effects on quality of life, functional status, prognosis and outcome. Associated Complications include:[13]
- Infections
- Chronic anemia
- Gastrointestinal cancers
- Pleural effusions
- Osteoporosis
- Heart disease
- Sicca syndrome
- Felty syndrome
- Lymphoma[1]
- Damage to the lung tissue (rheumatoid lung)
- Side effects from treatment and medication.
- General deconditioning
- Neurological complications
- Ocular complications
Diagnostic Procedures[edit | edit source]
The American College of Rheumatology has defined 7 criteria, where a patient has to correspond with at least 4 of these 7 criteria for the diagnosis of rheumatoid arthritis.The first 4 of these criteria are only valid if they persist for at least 6 weeks. These 7 criteria are:
- Morning stiffness
- Arthritis in 3 or more joints
- Arthritis in the joints of the hands (wrist, MCP, PIP)
- Symmetrical arthritis
- Rheumatoid nodules over bony prominences or extensor surfaces
- Positive serum rheumatoid factor
- Radiographic changes including erosion or bony decalcifications localized in or adjacent to joints.
| Test | Description | Purpose |
| C-reactive protein (CRP) (Lab Test) |
Blood Test | Indicates inflammation. As the level of inflammation rises, so does the level of CRP. High levels of CRP over a long period of time may indicate more severe joint damage. |
| Erythrocyte sedimentation rate, also referred to as ESR or "sed rate" (Lab Test) |
Blood Test | People with severe RA will generally have a higher ESR than one with less severe RA |
| Rheumatoid factor (RF) Lab Test |
Blood Test | Indicates the presence of RA. When the immune system attacks itself, the body produces RF. 75% of people with RA are RF positive. |
| Cyclic citrullinated peptide (CCP) Lab Test |
Blood Test | This test is usually given to people who are experiencing new symptoms of RA, in order to rule out other autoimmune diseases. |
| Bone Scans | Imaging | Detects inflammation |
| X-Ray | Imaging | Detects swelling of soft tissues and loss of bone density |
| Magnetic Resonance Imaging (MRI) | Imaging | Detects inflammation |
| Ultrasound | Imaging | Detects inflammation |
Prognosis[edit | edit source]
Rheumatoid arthritis has no cure and is a progressive disease. All individuals have multiple exacerbations and remissions. Close to 50% of patients with the disease become disabled within 10 years. Besides the joint disease, the individuals can suffer from many extra joint related problems which significantly alters the quality of life. The progression of disease does vary from individual to individual. The following factors determine worse prognosis:
- Elevated serum titer of autoantibodies
- Presence of HLA-DRB1*04 genotype
- Involvement of many joints
- Extra-articular features
- Female gender
- Age of less than 30
- Insidious onset
- Presence of systemic symptoms
Rheumatoid arthritis is also associated with cardiovascular risk factors, infection, respiratory disease and development of malignancies. Patients with rheumatoid arthritis have 2-3 times higher risk of death compared to the general population.[1]
Medical Management[edit | edit source]
A strategic approach is followed when managing rheumatoid arthritis, disease activity is assessed at regular intervals and treatment is changed as per the disease activity.
Disease-modifying, anti-rheumatic drugs (DMARDs) are initiated as soon as the diagnosis of rheumatoid arthritis is made.
- Traditional or conventional DMARD include methotrexate, leflunomide, sulfasalazine, hydroxychloroquine. Biologic DMARDs include TNF (tumor necrosis factor): Adalimumab, Etanercept, Infliximab, Golilumab, Certolizumab. And non-TNF inhibitors: Tocilizumab (Interleukin-6 inhibitor), Abatacept (inhibits T-cell costimulation), Rituximab (anti-B cell)
- Biological DMARDs (drug treatment), optimal outcome of treatment in rheumatoid arthritis (RA) is early clinical remission to delay joint damage. Therefore, severe RA patients with inadequate response to conventional disease modifying anti-rheumatic drugs (cDMARDs) need high-potency drug as biological DMARDs (bDMARDs). [14]
HMG-CoA reductase inhibitors (also known as statins) are widely used as lipid-lowering agents in patients with rheumatoid arthritis (RA) to reduce their cardiovascular risk. The Statin therapy also
- significantly reduced tender joint counts, swollen joint counts, erythrocyte sedimentation rate (ESR), compared with placebo groups.[15]
- influences immune regulation, potentially facilitating autoimmunity, eventually resulting in autoimmune diseases such as rheumatoid arthritis (RA)[16].
Radiosynovectomy, an intra-articular injection of small radioactive particles to treat a synovitis.
- The treatment can be repeated 3-time in an interval of 3 months if the first treatment showed an insufficient effect.
- Repeated treatments are more effective than single treatments with higher activity.
- The therapy is well-tolerated with a low rate of side effects.
Surgical treatment aimed at the inflammatory focus elimination and reduction of the pain syndrome severity, function loss, and the joint deformity. The most used operative interventions are tenonectomy, synovectomy, arthrodesis, total endoprosthesis.[17]
Nutritional Guidelines[edit | edit source]
Dietary interventions necessitate a widespread appeal for both patients as well as clinicians due to factors including affordability, accessibility, and presence of scientific evidences that demonstrate substantial benefits in reducing disease symptoms such as pain, joint stiffness, swelling, tenderness and associated disability with disease progression. However, there is still an uncertainty among the community about the therapeutic benefits of dietary manipulations for RA[18]. Dietary modification helps in staying in the remission phase of the inflammatory condition.
Eating certain foods can help you manage its symptoms. Dietary supplements[18] like vitamin D, cod liver oil, and multivitamins can also help in managing RA.
Avoiding food which causes inflammation like processed food, high salt, oils, butter, sugar, and animal products.
Supplements: Research suggests that there are vitamins and minerals which may have an effect on RA. These effects range from reducing joint inflammation to improving bone health. It is recommended to consult your primary care physician.
Physical Therapy Management[edit | edit source]
At present, there is no therapy that can completely heal RA. But there are treatments that achieve pain relief and the slowdown of the activity of RA to prevent disability and increase functional capacity. RA patients are unfortunately committed to a treatment for life.[19] The benefits of physical therapy interventions have been well documented.
Physical therapists play an integral role in the nonpharmacologic management of RA. They help patients with RA cope with chronic pain and disability through the design of programs that address flexibility, endurance, aerobic condition, a range of motion (ROM), strength, bone integrity, coordination, balance and risk of falls. All current UK clinical guidelines for the management of RA recommend the use of physiotherapy (PT) and occupational therapy (OT) as an adjunct to drug treatment. The three most common components of PT/OT for RA hands are exercise therapy, joint protection advice and provision of functional splinting and assistive devices, massage therapy, exercise therapy and patient education. Dynamic exercise (aerobic capacity and/or muscle strength training) was effective in improving muscular endurance and strength, without detrimental effects on disease activity or pain.
The therapy goals in most cases are: [20]
- Improvement in disease management knowledge
- Pain control
- Improvement in activities of daily living
- Improvement in Joint stiffness (~ Range of motion)
- Prevent or control joint damage
- Improve strength
- Improve fatigue levels
- Improve the quality of life
- Improve aerobic condition
- Improve stability and coordination
Patient questionnaires – not joint counts, radiographic scores, or laboratory tests – provide the most significant predictors of severe 5-year outcomes in patients with RA, including functional status, work disability, costs, joint replacement surgery and premature death.
Physiotherapy Modalities[edit | edit source]
• Cold/Hot Applications: cold = for acute phase; heat = for chronic phase and used before exercise. Dose and/or withdraw thermal energy by means of hot/cold application. [21]
• Electrical Stimulation: Aperitif administering of electric energy by means of an alternating current. Transcutaneous electrical nerve stimulation (TENS) is used to relieve pain. [22]
• Hydrotherapy-Balneotherapy: allows exercise with minimal load on the joints.
Simply being in another environment, where the patient can relax has a positive effect on the disease's progression (physically as well as on mentally)[23] The evidence for Balneotherapy is positive but lacks trials undertaken with robust methodologies. [24]
Rehabilitative Treatment[edit | edit source]
Joint Protection Strategies:[edit | edit source]
- Rest & Splinting: Orthosis and splinting prevent the development of deformities and support joints
- Therapy Gloves: to control and manage hand pain, to maintain or restore the patient’s hand function, or to psychologically help to relax or calm the wearer. Wearing therapy gloves led to the improvement in hand grip strength. The glove can be worn during the day or at night. They are made of various materials: nylon, wool and elastane fibres. [25]
- Compression Gloves: moderate joint swelling and consequently reduce the pain
Assistive Devices and Adaptive Equipment:[edit | edit source]
Arrangements (like elevated toilet seats,...) to facilitate activities of daily living
Massage Therapy[edit | edit source]
Massage and the manual trigger of an articular movement focused on the improvement of function, pain reduction, reduction of disease activity improve flexibility and welfare (dimension of depression, anxiety, mood and pain) [26]
Therapeutic Exercise[edit | edit source]
Physical exercise helps to increase the physical capacity of the patient but it does not reduce the activity of the disease[19]. There is evidence suggesting that exercise improves general muscular endurance and strength without detrimental effects on disease activity or pain in rheumatoid arthritis. However, few studies have investigated the effect of exercises for the rheumatoid hand. Some improvement in strength, mobility and/or function with no negative effects have been reported, although the long-term effectiveness has not been established due to various weaknesses in trial design.[27] The duration and the frequency of the treatment depend on the perceived limitations in activities and participation, and the impairments in functions and structures.[28][29]
Before beginning an exercise program, it is important to have a global evaluation of the situation: joint-inflammation local or systemic, state of the disease, age of the patient and grade of collaboration.[19]
Exercise therapy is used by patients with RA with the aim of improving daily functioning and the social participation by means of improvement of the strength, aerobic condition, the range of motion, stabilization and coordination.
In general, we can say that patients with RA need a high-intensive exercise program which is aimed at improving aerobic capacity, strength and endurance. This program can be completed with ROM-exercises and stabilization/coordination exercises. Sometimes the therapist chooses to start with a moderate-intensive exercise program and built this up. This is often the case in patients with joint prostheses, severe physical disabilities and/or kinesiophobia. [30][31] The duration and intensity of the exercises should be based on the individual patient and their assessment[19].
Precautions[19][edit | edit source]
- When the patient experiences an exacerbation and the joints are acutely inflamed then isometric exercises should be done
- Avoid stretching in acute cases.
- Revise the exercise program if pain persists 2 hours after the activity or there is an increase in joint swelling
- Patients with active RA in their knees should avoid climbing stairs or weight lifting as it could lead to intra-articular pressure in the knee joint
- Avoid excessive stress over the tendons with stretches and avoid ballistic movements
Exercises[edit | edit source]
- ROM exercises: In acute phase: isometric/static exercises -> be held for 6 seconds and repeated 5–10 times each day ; load = 40% 1RM. in chronic phase: isotonic exercises (= active exercises with constantly the same tension) for example: swimming, walking, cycling -> minimum 4 repetitions for each joint in 2 to 3 days These exercises increase the mobility of the joint, but the concerned joint will not be loaded during this exercises.[32] Contractures can be held for 6seconds and repeated 5-10 times daily[19].
- Stretching: Has to be avoided in acute cases.
- Strengthening: Moderate-intensive exercise therapy where a minimum of 8-10 exercises is necessary for the major muscle groups. Each exercise has to be repeated 8-10 times and a minimal start intensity of 30-50 percent of 1 repetition maximum (RM). [31] [32] Use light weights important for stabilization of the joint and prevention of traumatic injuries.
- Aerobic condition exercises: There are two types of exercises to improve the aerobic condition: Intensive exercises and moderate-intensive exercises. The intensive exercise therapy has a minimum duration of 20 minutes per session and this 3 times a week with an intensity of 65 to 90 percent of the maximal heart rate. The moderate-intensive exercise therapy has a minimum duration of 30 minutes per session and this 5 times a week with an intensity of 55 to 64 percent of the maximal heart rate. The aim of this exercises is to improve the muscle endurance and aerobic capacity.
- Stabilizing and coordinating exercises: The improvement of stabilization and coordination of a certain joint will be achieved by doing exercises that stimulate the sensorimotor system. For example, standing on a balance board. Important aspects during this exercises are motion control, balance and coordination.
- Routine daily activities: SARAH (Strengthening and stretching for rheumatoid arthritis of the hand) exercise program: The SARAH trial tests an intervention against the usual hand care. The main aim of the exercise program is increased hand function, which is suggested to be mediated by increases in strength, dexterity and range-of-movement. The exercise program consists of the usual care plus a hand and wrist exercise program which includes seven mobility exercises and four strength exercises against resistance (i.e. therapy putty, theraband or hand exerciser balls).[10]
- Conditioning exercises in people with chronic inactive RA: swimming walking, cycling (include adequate rest periods)[19].
| Exercises | Frequency | Sets | Repetitions | Initial Hold | Initial Load | Progression | |
| Mobility | • MCP flexion • Tendon gliding • Finger radial walking • Wrist circumduction • Finger adduction • Hand-behind-head • Hand-behind-back |
Daily | 1 | x 5 | 5 seconds (where required) | Step 1: increase up to 10 repetitions Step 2: Increase up to 10 seconds hold | |
| Strength | • Eccentric wrist extension • Gross grip Finger adduction • Pinch grip |
Daily | 1 | X 8 (min. 8 repetitions, max. 12 repetitions) | Between 3 and 4 on modified 10 pt Borg Scale | Step 1: 2x 10 repetitions Step 2: 4-5 on Borg scale Step 3: 5-6 on Borg scale Step 4: 3x 10 repetitions |
A modified Borg scale is used to set the load (resistance) for the strength exercises based on self-perception of effort. The level of resistance is determined by the patients’ rating of perceived effort using the weaker hand for each strength exercise. Exercise therapy in patients with RA is used to improve the daily functioning and social participation through improving muscle strength, aerobic endurance, joint mobility (range of motion, ROM) and stability and/or coordination. Therefore, preference is given to an active policy, especially where the physiotherapist has a supporting role. However, in individual cases, passive treatments, such as manual operations, can be part of the treatment.
5. Patient Education: information about their condition and the different therapies disposed to improve their quality of life. In addition, patients are taught how to protect the joints during routine daily life. To let patients become more active, you have to adjust their movement-behaviour. Different manners to achieve a behavioural change by your patient:
• A behavioural change is a process with 3 phases: the motivation-phase, the initial-behavioural change phase and the phase where the intended behaviour is continued. It’s important to know in which phase your patient is located. In the 1st phase is education and motivation important. In the 2nd phase is structure and accompaniment important and in the 3rd phase is the patient capable to keep moving with the help of his natural environment.
• The therapist has to formulate achievable goals with the patient.
• The therapist should give proper instructions and be sure that the patient understands him.
• Enough variation in the exercises is important. Otherwise, the patient can become bored which is not positive for your therapy.
• The therapist needs to avoid that the patient gets again in his old movement-pattern. You must warn the patient for this.
• The therapist has to involve the partner and other important people in the process because they have an important motivation-role. Also, the therapist himself has to motivate the patient.
• Keep in touch with the patient to be sure that the treatment was effective. [33]
Adult Rheumatoid Arthritis Physical Therapy Practice Patterns
4A: Primary Prevention/Risk Reduction for Skeletal Demineralization (low bone density)
4B: Impaired Posture (cervical involvement)
4C: Impaired Muscle Performance
~4D: Impaired Joint Mobility, Motor Function, Muscle Performance, and Range of Motion Associated with Connective Tissue Dysfunction
4H: Impaired Joint Mobility, Motor Function, Muscle Performance, and Range of Motion Associated with Joint Arthroplasty
4I: Impaired Joint Mobility, Motor Function, Muscle Performance, and Range of Motion Associated with Bony or Soft Tissue Surgery ( tenosynovectomy, tendon reconstruction)
5H: Impaired Motor Function, Peripheral Nerve Integrity, and Sensory Integrity Associate with Nonprogressive Disorders of the Spinal Cord (cervical spine)
6B: Impaired Aerobic Capacity/Endurance Associated with Deconditioning
~Most Commonly used practice pattern for RA.
Management of flare ups
People who are diagnosed with RA also may experience a phenomenon that is called an “flare up” of the condition. Research suggests that these flare ups usually happen after experiencing a secondary illness, being involved in a high stress situation, overexerting oneself, . What triggers flare ups is currently still unknown. Current evidence suggests some strategies that can help someone who is experiencing a flare up by reducing pain, inflammation, and improve their quality of life:
- Balance is key, schedule plenty of down time to reduce the likelihood of affected joints from becoming flared up
- Educate family, staff at work, and other people who you interact with, they can help you during flare ups
- Have a backup plan, be prepared in case of a flare up and become familiar with warning signs of a flare up
- Practice relaxation and self calming strategies: Research suggests that regularly practicing these relaxation techniques can reduce stress and lead to a reduction in pain.
- Use modalities such as a cold pack or hot pack: Both of these have various effects on tissues which research suggests can be beneficial in reducing inflammation and pain during a flare up
- Lastly, corticosteroid injections can be used to reduce inflammation and reduce pain in a flared up joint
Outcome Measures[edit | edit source]
- Rheumatoid Arthritis Disease Activity Index (RADAI-5): A self-reported outcome measure that consists of 5 questions in a Likert scale format that briefly surveys the patient regarding their views of their condition (both over the past 6 months and current status).
- Disabilities of the Arm, Shoulder and Hand (DASH): The DASH is a patient-reported outcome measure that evaluates function of the upper extremities, and can be used to examine change over time.
- Short Form-36 (SF36): A patient reported outcome measure that is designed to evaluate quality of life through measures such as physical functioning, role limitations due to physical or emotional problems, and general mental health.
- Fatigue Severity Scale: A 9-item questionnaire that rates the patient’s fatigue and how it interferes with activities such as work or social life.
The Multi-Dimensional Health Assessment Questionnaire (MDHAQ) is a recognized quality-of-care indicator. Assesses pain and fatigue using visual analogue scales (VASs) and includes items to assess disability in these patients.
The Multi-Dimensional Health Assessment Questionnaire (MDHAQ) derived from the HAQ (=a disease-specific questionnaire), which includes an index of the three RA core data set measures (physical function, pain, and global estimate), also known as a routine assessment of patient index data 3 (RAPID 3). The MDHAQ is created for the clinical standard care to save time for the rheumatologist and to improve the quality of patient visits.
The difference between the HAQ and MDHAQ are:
- The MDHAQ has two activities more than the HAQ – “Are you able to walk 2 miles or 3 kms?” and “Are you able to participate in recreation and sports as you would like?”. These ADLs were added as scores for eight items on a modified HAQ (MHAQ) and were systematically lower than HAQ scores by 0.2 units to 0.3 units. Scores on the HAQ and MDHAQ are quite similar. Inclusion of two complex activities reflects higher expectations for patient status in rheumatology care at this time than in the 1970s when the HAQ was developed.
- All 10 activities are listed on one side of the first page, allowing the physician or other health professionals to scan the information rapidly.
- The MDHAQ does not include HAQ queries concerning aids, devices, or help from another person, which complicates scoring, may not add important information (particularly at this time), and possibly elevate scores artifactually with use of a device.
- The MDHAQ VAS for pain and patient global estimate are in a format of 21 numbered circles, rather than a 10-cm line and require no ruler to score.
- The MDHAQ includes a patient self-report RA disease activity index (RADAI) joint count.
- Boxes are available on the MDHAQ to record scores for physical function, pain, patient global estimate, and RADAI self-report joint count.
- Scoring templates are available on the MDHAQ to convert physical function scores from 0-30 to a 0-10 scale, and RADAI self-report joint counts scores from 0-48 to a 0-10 scale.
- Scoring templates are also available to record RAPID composite scores. RAPID 4 adds a RADAI self-report joint count and RAPID 5 adds a physician global estimate.
- The MDHAQ also includes three psychological items concerning sleep, anxiety, and depression (queried in the standard patient-friendly HAQ format), not scored formally, a review of systems, medical history, fatigue VAS, queries about change in status, morning stiffness and exercise, and demographic data—within two sides of one page.
Two prerequisites are essential for success in having patients complete questionnaires:
- The questionnaire must be reviewed by the rheumatologist prior to seeing the patient, so the staff and patients recognize that this is an important matter and not simply an exercise to meet abstract goals or requirements for a clinical study (as is the situation in completion of questionnaires in many research studies) or requirements to administer a certain therapy.
- The staff must project an attitude of enthusiasm, reflecting the interest of the clinician. For example,
a comment such as “Would you mind completing a questionnaire?” is inappropriate. A better comment might be: “We need you to complete this questionnaire as part of your medical evaluation.”
The MDHAQ is useful in all rheumatic diseases by documenting changes in status over long periods, and by improving rheumatology care and outcomes. .
Goals of the MDHAQ:
- to be scanned (“eyeballed”) by a clinician in 5 sec to 10 sec
- using scoring templates on the questionnaire for individual measures in less than 10 sec or 10 sec
- RAPID indices based on self-report data.
All quantitative data require interpretation by a clinician, along with information from a history, physical examination, and other sources in formulating a clinical decision. Nonetheless, the availability of quantitative data can add considerably to the decision process and help focus the visit on the concerns of the patient.
Classification of Functional Status[edit | edit source]
The American College of Rheumatology classified functional status in Rheumatoid Arthritis as:
- Class I: Completely able to perform usual activities of daily living (self-care, vocational, and avocational)
- Class II: Able to perform usual self-care and vocational activities, but limited in avocational activities
- Class III: Able to perform usual self-care activities, but limited in vocational and avocational activities
- Class IV: Limited ability to perform usual self-care, vocational, and avocational activities
Resources[edit | edit source]
- Rheumatoid Arthritis: Help to understand Rheumatoid Arthritis
- Rheumatoid Arthritis: Frequently asked Questions
- Rheumatology Check List Visit
- The RA Symptom Tracker Sheet
- The Arthritis Organization
- American College of Rheumatology Patient Education
- Self-help (Aids for Arthritis)
- RA Treatments
- American College of Rheumatology
Clinical Bottom Line[edit | edit source]
Rheumatoid arthritis (RA) is a systematic autoimmune inflammatory disease and results in persistent inflammation of synovial tissue especially of the wrists, hands and feet. There is no therapy that can completely heal RA. But there are treatments that achieve pain relief and the slowdown of the activity of RA to prevent disability and increase functional capacity. Physical therapists play an integral role in the nonpharmacologic management of RA. They help patients with RA cope with chronic pain and disability through the design of programs that address flexibility, endurance, strength, bone integrity, coordination, balance and risk of falls.
References[edit | edit source]
- ↑ 1.0 1.1 1.2 1.3 1.4 1.5 1.6 1.7 1.8 1.9 Krati Chauhan; Jagmohan S. Jandu; Mohammed A. Al-Dhahir. Oct 2019 RA Available from:https://www.ncbi.nlm.nih.gov/books/NBK441999/ (last accessed 23.2.2020)
- ↑ Ottawa Methods Group, Ottawa Panel Evidence-Based Clinical Practice Guidelines for Therapeutic Exercises in the Management of Rheumatoid Arthritis in Adults, Phys Ther. 2004;84:934–972 (Level 1A).
- ↑ 3.0 3.1 3.2 3.3 KNGF-richtlijn. Reumatoïde artritis. 2008
- ↑ Maura D. Iversen et. Al, Predictors of the use of physical therapy services among patients with rheumatoid arthritis © 2011 American Physical Therapy Association, Issue 91, pages 65-67 (Level 2B )
- ↑ Arthritis Foundation. Disease fact sheet: http://www.arthritis.org/conditions-treatments/disease-center/rheumatoid-arthritis/ (accessed 25 February 2013)
- ↑ CDC. Rheumatoid Arthritis.http://www.cdc.gov/arthritis/basics/rheumatoid.htm (accessed 13 February 2013)
- ↑ 7.0 7.1 Demoruelle MK, Deane KD, Holers VM. When and where does inflammation begin in rheumatoid arthritis?. Current opinion in rheumatology. 2014 Jan;26(1):64.
- ↑ 8.0 8.1 8.2 8.3 8.4 8.5 8.6 8.7 Deane KD, Demoruelle MK, Kelmenson LB, Kuhn KA, Norris JM, Holers VM. Genetic and environmental risk factors for rheumatoid arthritis. Best Practice & Research Clinical Rheumatology. 2017 Sep 18.
- ↑ Alpízar-Rodríguez D, Pluchino N, Canny G, Gabay C, Finckh A. The role of female hormonal factors in the development of rheumatoid arthritis. Rheumatology. 2016 Sep 29;56(8):1254-63.
- ↑ 10.0 10.1 SARAH Trial Team et al., Strengthening and stretching for rheumatoid arthritis of the hand (SARAH): design of a randomised controlled trial of a hand and upper limb exercise intervention - ISRCTN89936343, Trial Team et al. BMC Musculoskeletal Disorders 2012, 13:230 (Level 1A)
- ↑ Geri B. Neuberger et al., Predictors of exercise and effects of exercise on symptoms, function, aerobic fitness, and disease outcomes of rheumatoid arthritis. 2007, 943-952
- ↑ Pubmed. Comorbidities in rheumatoid arthritis. http://www.ncbi.nlm.nih.gov/pubmed/17870034 (accessed 12 February 2013).
- ↑ Gabriel SE. Cardiovascular morbidity and mortality in rheumatoid arthritis. Am J Med. 2008 Oct;121(10 Suppl 1):S9-14.
- ↑ Wacharapornin P, Suwannalai P, Predictors for low disease activity and remission in rheumatoid arthritis patients treated with biological DMARDs, J Med Assoc Thai. 2014 Nov;97(11):1157-63,
- ↑ Xing B. et al., Effect of 3-hydroxy-3-methylglutaryl-coenzyme a reductase inhibitor on disease activity in patients with rheumatoid arthritis: a meta-analysis.
- ↑ de Jong HJ, Klungel OH, Van Dijk L, Vandebriel RJ, Leufkens HG, van der Laan JW, Tervaert JC, Van Loveren H. Use of statins is associated with an increased risk of rheumatoid arthritis. Annals of the rheumatic diseases. 2011 Oct 1:annrheumdis-2011.
- ↑ (No authors listed) Affection of radio-carpal joint in patients with rheumatoid arthritis and its surgical treatment, Klin Khir. 2014 Aug;(8):65-9.
- ↑ 18.0 18.1 Khanna S, Jaiswal KS, Gupta B. Managing Rheumatoid Arthritis with Dietary Interventions. Frontiers in nutrition. 2017 Nov 8;4:52.
- ↑ 19.0 19.1 19.2 19.3 19.4 19.5 19.6 Vural Kavuncu, MD* and Deniz Evcik, MD Physiotherapy in Rheumatoid ArthritisfckLRMedGenMed. 2004; 6(2): 3.
- ↑ Marry J. Bell et al., A Randomized control trial to evaluate the efficacy of community based physical therapy in the treatment of people with rheumatoid arthritis, 1998 Feb;25(2):231-7. (Level 1B)
- ↑ Bijlsma J.W.J., Geusens P.P.M.M., Kallenberg C.G.M., P.P. Tak. Reumatologie en klinische immunologie. Houten: Bohn Stafleu van Loghum 2004. (level 5)
- ↑ Pelland L, Brosseau L, Casimiro L, Robinson VA, Tugwell P, Wells G. Electrical stimulation for the treatment of rheumatoid arthritis. The Cochrane database of Systematic Reviews. 2002;2:CD003687. (Samenbundeling van zowel RCT’s, cohortstudies als case-control studies dus level of evidence: 1, 2 en 3)
- ↑ Angela Reid1 , Audrey Brady1 , Catherine Blake2 , Anne-Barbara Mongey3 , Douglas J Veale3 , Oliver FitzGerald3 and Tara Cusack2fckLRRandomised controlled trial examining the effect of exercise in people with rheumatoid arthritis taking anti-TNFα therapy medicationfckLRBMC Musculoskeletal Disorders 2011, 12:11doi:10.1186/1471-2474-12-11 (level 1B)
- ↑ Verhagen AP, Bierma-Zeinstra SM, Cardoso JR, Bie RA de, Boers M, Vet HC de. Balneotherapy for rheumatoid arthritis. The Cochrane Database of Systematic Reviews. 2003;4:CD00518. (Level 1A)
- ↑ Nasir SH, Troynikov O, Massy-Westropp N. Therapy gloves for patients with rheumatoid arthritis: a review. Therapeutic Advances in Musculoskeletal Disease. 2014;6(6):226-237. doi:10.1177/1759720X14557474. (level 3A)
- ↑ Brownfield A. Aromatherapy in arthritis: a study. Nurs Stand. 1998;13;5:34-35. (Level1)
- ↑ Heine P.J. et al., Development and delivery of an exercise intervention for rheumatoid arthritis: Strengthening and stretching for rheumatoid arthritis of the hand (SARAH) trial. Physiotherapy 98 (2012) 121-130 (Level 1B)
- ↑ Minor MA, Hewett JE, Webel RR, Anderson SK, Kay DR. Efficacy of physical conditioning exercise in patients with rheumatoid arthritis and osteoarthritis. Arthritis Rheum. 1989;32;11:1396-1405. (Level 1B)
- ↑ Ende CH van den, Hazes JMW, le Cessie S, Mulder WJ, Belfor DG, Breedveld FC, et al. Comparison of high and low intensity training in well controlled rheumatoid arthritis. Results of a randomized clinical trial. Ann Rheum Dis. 1996;55;11:798-805. (Level 1B)
- ↑ Jong Z de, Munneke M, Zwinderman AH, Kroon HM, Jansen A, Ronday KH, et al. Is a long term high intensity exercise program effective and safe in patients with rheumatoid arthritis? Results of a randomized controlled trial. Arthritis Rheum. 2003;48;9:2415-24. (Level 1B)
- ↑ 31.0 31.1 Ende CH Van den, Vliet Vlieland TP, Munneke M, Hazes JM. Dynamic exercise therapy for rheumatoid arthritis. Cochrane Database Syst Rev 2000;(2):CD000322. (Level 1A)
- ↑ 32.0 32.1 Minor MA, Hewett JE, Webel RR, Anderson SK, Kay DR. Efficacy of physical conditioning exercise in patients with rheumatoid arthritis and osteoarthritis. Arthritis Rheum. 1989;32;11:1396-1405. (Level 1B)
- ↑ Brodin N, Eurenius E, Jensen I, Nisell R, Opava CH, PARA study group. Coaching patients with early rheumatoid arthritis to healthy physical activity: a multicenter, randomized, controlled study. Arthritis Rheum. 2008;59;3:325-31. (Level 1B)
- ↑ O’Sullivan and Schmitz. Physical Rehabilitation. 5th edition. Philadelphia, PA: F.A. Davis Company. 2007.