Breast Cancer
Top Contributors - Adam El-Sayed, Kim Jackson, Lucinda hampton, Vidya Acharya, Admin, 127.0.0.1, Chee Wee Tan, Ilona Malkauskaite, Candace Goh and Manisha Shrestha - Ashlea Anthony & Linsey Schmalz from Bellarmine University.
Introduction[edit | edit source]
Female breast, lung and colorectal cancer account for one-third of the world's cancer incidences.[1]
When looking at cancer mortality across the globe female breast cancer is ranked 5th in terms of mortality.[1] However, with the advances in both the diagnostic and treatment approaches for breast cancer survival rate after a diagnosis of breast cancer has significantly improved.[2] The increase in the number of breast cancer survivors has resulted in more research and care being directed toward developing interventions that will help improve the overall quality of life for women who have survived breast cancer.[3]
Physiotherapists have an important role in the rehabilitation process during and after a diagnosis of breast cancer, as well as in the care of survivors.
What is Breast Cancer?[4][edit | edit source]
Breast cancer involves the production of malignant cells in the breast. These cells grow uncontrollably affecting nearby tissue and/or spreading to other parts of the body through a process called metastasis.
However, not all cell growth in breast tissue is malignant. Non-cancerous conditions such as atypical hyperplasia cysts and abscesses, and non-cancerous tumours such as intraductal papillomas can form.[4]
Breast cancers are usually adenocarcinomas. These tumours develop in the following two locations within the breast:
- Ductal carcinoma: The cells that line the milk ducts
- Lobular carcinoma: The milk-producing glands
Ductal and lobular carcinomas can each be classified as in-situ or invasive (infiltrating). With in-situ, there is no growth into surrounding tissues (the cancer is confined within the ducts or lobules). With invasive, the malignant cells that started in the ducts or glands have begun to invade the surrounding tissue and potentially other parts of the body.[5]
Metastases[edit | edit source]
As previously mentioned, metastasis involves the spread to one or more sites elsewhere in the body. This occurs by way of directly affecting an organ or travelling through the lymphatic and/or circulatory systems.[6]
The following terms can be utilized to classify how far the malignant cells have spread:[7]
- Localized means there is no spread.
- Regional means there is spread to the lymph nodes, tissues, or organs close to where cancer started (the primary site).
- Distant (also known as metastatic cancer) means there is spread to organs or tissues that are farther away from the primary site. The main sites of metastasis for breast cancer include bones, lungs, brain, and liver.[8]
Other types of breast cancer[edit | edit source]
Other types of breast cancer are not nearly as common as the adenocarcinomas. They include the following:[4]
- Inflammatory breast cancer
- Paget disease of the nipple
- Triple negative breast cancer
Staging[4][5][edit | edit source]
The system commonly used to classify the extent of breast cancer is the AJCC/TNM system.
The TNM system uses information on:
- T: tumour size and how far it has spread within the breast and nearby organs
- N: lymph node involvement
- M: the presence or absence of distant metastases
Once the T, N, and M are determined through stage grouping, a stage of 0, I, II, III, or IV is assigned.The stage number and degree of cancer spread are positively correlated.
Review the stages of breast cancer.
Pathophysiology in Breast Cancer[10][edit | edit source]
- Non-invasive breast cancers are present in the ducts or lobules.
- Invasive cancers are found in the surrounding breast tissue.
- Cell Grade: Cells are graded in a system where grade 1 cancer cells present slightly differently to normal cells, progressing to grade 3 cancer cells which demonstrate major differences to a normal cells.
- Tumour Necrosis: tumour necrosis may be present in aggressive forms of breast cancer where cells are seen to grow at a rapid rate.
- This is often a sign of a rapidly growing aggressive form of breast cancer.
- Vascular or Lymphatic Invasion: - these types of invasion describe whether or not cancerous cells are evident in the vascular and lymphatic vessels supplying the breast tissue.
- Hormone Receptor Status: - Hormone receptor status determines if hormone therapy would be appropriate.
- HER2 Status: - HER2 is a gene that when dysfunctional can play a role in the development of breast cancer. Breast cancers that are HER2 positive tend to grow faster and are more likely to spread that those that are HER2 negative.
Epidemiology[edit | edit source]
Breast cancer can occur in both men and women.
However, it is quite rare in men; it accounts for 1% of cancers and 0.2% of cancer-related deaths in men.[11][12][13][14][15][16][17][18]
One in 8 women will develop invasive breast cancer in their lifetime and approximately 1 in 33 will die from it.[19] In 2018, approximately 2.1 million women received a diagnosis of breast cancer.[20] This makes it the most commonly diagnosed cancer and the second leading cause of cancer-related deaths in women.[6]
There has been an increase in breast cancer incidence and related mortality by nearly 18 % since 2008. The reason for this may be due to a combination of advances in screening and diagnosis, as well as increased rates of obesity and fewer child births per woman.[20][21]
Survivor-ship varies across the globe, such that 5-year relative survival was ≥80% in the United States, Canada, and Austria, but <40% in Denmark, Poland, and Algeria.[22] This may be attributed to differences in diagnostics and treatments,as well as a lack of healthcare resources in some countries[23][24][25]
Breast cancer-related lymphoedema (BCRL) is condition that a woman can develop anytime 3-20 years after treatment.[26] The incidence varies and likely depends on the type of treatment received. Recent evidence suggests that 1 in 5 women will acquire it at some point.[27]
Risk Factors[edit | edit source]
Age[edit | edit source]
Older age has been shown to increase the risk of developing breast cancer.[28][29] Between 2013 and 2015, the probability of a woman developing breast cancer in the United States between birth to 49 years of age was 1 in 51; the probability increased when ≥70 years of age to 1 in 15.[28]
Sex[edit | edit source]
Females are more at risk then males
Family history/ past medical history[edit | edit source]
Personal history of breast cancer. Family history of breast cancer: In comparison to women who have do not have a family history of breast cancer, one first-degree relative has been found to lead to a 1.75-fold higher risk and 2 first-degree relatives has been found to lead to a 2.5-fold higher risk of developing breast cancer.[30]
Breast cancer-associated genes 1 and 2 (BRCA1 and BRCA2) mutations: BRACA1 and BRCA2 are responsible for the suppression of tumours.[31] Furthermore, mutations of these genes have been shown to increase the risk of developing breast cancer.[32]
Race: Non-hispanic white women show a higher incidence of breast cancer.[33] African-American, Hispanic, Native American and Asian women show a higher mortality rate.[33]
Lifestyle[edit | edit source]
Alcohol consumption has been shown to be a risk factor for the development of breast cancer.[34][35][36][37] 10 g more of alcohol per day may increase the risk by approximately 7.1%.[36]
Obesity, especially in postmenopausal women, has been found to increase the risk of developing breast cancer.[38] (30 and 31)
Cigarette smoking: There is no consensus in the evidence that cigarette smoking increases the risk of developing breast cancer, which may be partly because of the correlation between smoking and alcohol consumption.[39] An analysis[36] found no significant difference between smokers and non-smokers.[36] However, Terry and colleagues (2002)[40] found that those who smoked more and for longer were at a higher risk, especially if they smoked 20 or more cigarettes per day over the course of 40 years or more.[40]
- Menstrual history: A menstrual period that starts early and ends later in life may increase the risk of developing breast cancer.[29]
- Childbirth: Having children after the age of 30 or not having any children at all may increase the risk of developing breast cancer.
- Endogenous and exogenous estrogens: Exogenous estrogens normally come from oral contraceptives and hormonal replacement therapy.[41] A combined oral contraceptive, one that includes both estrogen and progesterone, may increase the risk of the development of breast cancer, but this increased risk did not remain 10 years after stopping the pill.[39][42] In the UK, the Million Women Study found an increased risk in the development of breast cancer after using estrogen and progesterone combinations in hormone replacement therapy.[43] However, this risk did not remain 2 years after stopping hormone replacement therapy.[44]
See The National Cancer Institute Breast Cancer Risk Assessment Tool
Clinical Presentation[edit | edit source]
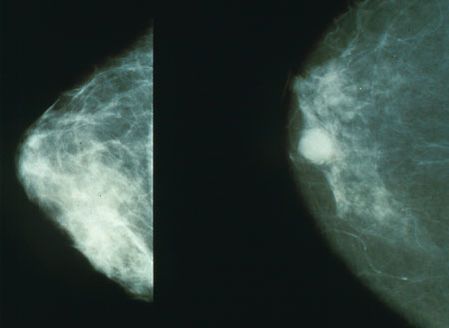
Breast cancer may be asymptomatic and undetectable in its earlier stages. The hallmark signs and symptoms of a ductal carcinoma are a lump in the breast and breast tenderness (not usually pain). The hallmark signs and symptoms of a lobular carcinoma do not involve a lump. There is often a change in breast texture. Therefore, a lobular carcinoma may be harder to detect.[45]
Other signs and symptoms of breast cancer may include the following:[4]
- Change in breast shape or size[4]
- Unusual discharge from the nipple[4]
- Lump in armpit[4]
- Retraction inwards or inversion of the nipple[4]
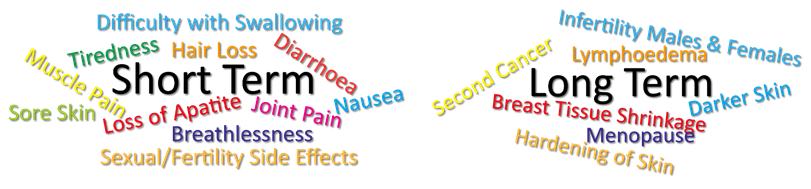
Late signs and symptoms of breast cancer may include the following:[4]
- Bone pain[4]
- Weight loss[4]
- Nausea[4]
- Loss of appetite[4]
- Jaundice[4]
- Shortness of breath[4]
- Cough[4]
- Headache[4]
- Double vision[4]
- Muscle weakness[4]
A useful mnemonic to help remember some of the signs of breast cancer is B.R.E.A.S.T.[46]
Musculoskeletal Problems Experienced in Breast Cancer Patients[edit | edit source]
The breast cancer patient can also be susceptible to the development of musculoskeletal problems, the same as the general population. One common problem is symptomatic rotator cuff disease, which can be brought on through intrinsic factors such as age related physiological changes to the tendons, or through extrinsic factors brought on from cancer treatment such as lymphedema as well as shoulder girdle resting alignment. Tension overload on the rotator cuff tendons may be increased secondary to increased volume and weight of the effected limb with the presence of lymphedema. Due to pain, or fear of movement, for example, the breast cancer patient may adapt to a new resting position for their shoulder, and may tend to avoid using the limb, resulting in shortening of the muscles, and tightening of the joint capsule [47]. Moreover, patients tend to adapt a flexed and protective posture following surgery, further increasing the likelihood of muscle shortening. Pectoralis major is commonly effected. Tightness of these muscles tend to lead to a pull on the scapula, causing it to become protracted and depressed, leading to scapular winging, as well as shoulder impingement [48].
Post-Mastectomy Pain Syndrome (PMPS)[edit | edit source]
Pain which lasts longer than what is usually expected following various breast cancer surgery types. Generally neuropathic in nature, and can be due but not limited to:
- Brachial nerve damage, *Intra-operative compromise of cutaneous innervating,
- Neuroma formation,
- Fibrotic entrapment. Patients often report neurological symptoms such as numbness or pins and needles, stabbing and burning pain to the same side as surgery in or around the surgical sites.
These symptoms can be exacerbated through a lack of pacing, or by lying on the side of surgery. Therefore, patient education, soft tissue massage, and other desensitising techniques are essential [48]. Other common problems include:
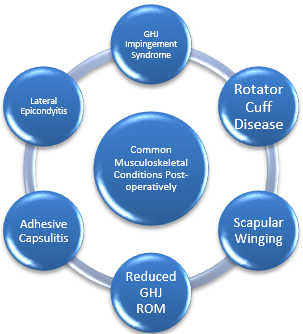
- Subacromial Impingement Syndrome.
- Adhesive Capsulitis (frozen shoulder) – idiopathic or traumatic (post-surgery).
- Rotator Cuff pathology (e.g Symptomatic Rotator Cuff Disease)
- Myofascial Dysfunction Lateral epicondylitis.
- Scapular winging secondary to damage of long thoracic nerve during surgery.
- Pain
Associated Neuromusculoskeletal Conditions Post Treatment[edit | edit source]
Neuromusculoskeletal conditions are common following surgery, some of which are illustrated in figure 1.4. Treatment protocols shall not be discussed, and the reader should refer to the basic principles of rehabilitation of musculoskeletal conditions. In light of this, it is important to briefly discuss a few points to consider.
- Depending on the type of surgery that the patient needs to undertake, radiotherapy may be necessary following surgery.
- A typical radiotherapy session will require the patient to position the treated arm to 90° flexion and abduction, as well as maximal external rotation, for up to 30 minutes [49].
- Shoulder mobility is commonly affected post-surgery [50]; [51]; [52] so it is vital that physiotherapy aims to restore this to improve patient functional ability and to be able to place the shoulder in the required positions for radiotherapy.
- Active, active assisted, and passive ROM exercises for the shoulder girdle are therefore good practice. Physiotherapy should aim to restore full shoulder ROM as well as minimising associated upper extremity morbidity [53].
- Manual therapy techniques with the aim of further increasing available ROM have been shown to not be of any significant benefit when used in conjunction with active upper limb exercises [54].
Breast Self-Examination[edit | edit source]
There does not seem to be a consensus on whether or not breast self-examinations (BSE) should be carried out by women.[56] In fact, the Canadian Taskforce on Preventive Health Care and the American Cancer Society (ACS) no longer recommend BSE.
Allen et al (2010)[57] commented on the potential benefits and harms of BSE. Some of the benefits included that BSE increases a woman's sense of comfort and autonomy with their health and breasts, the tool is non-invasive, lumps can be palpated, and women become more aware of breast changes over time.[57] Some of the harms included the potential for increased usage of the healthcare system and its resources (more cost, biopsies, etc), as well as higher levels of anxiety depression.[57] Furthermore, women should speak with their physicians to discuss whether the benefits of BSE outweigh the risks for that individual and that they seek clarity on how to perform the examination.
In conclusion, it remains important that women become familiar with the appearance and feel of their breasts. Any changes that may be detected can be reported to a physician for further investigation.
Diagnosis[edit | edit source]
- Ultrasound: An ultrasound may be performed if a lump is suspected, and this test creates a picture of the tissue within the breast. Ultrasounds can help determine if the area in question is a cyst or a solid lump.[58][59]
- IR thermography: It is a powerful tool that is also non-invasive and non-intrusive easing the analysis, providing safety and comfort to the patients. It can be used in women of different ages and health conditions without any risk[60].
- Mammography: A mammogram provides an x-ray of the breast tissue. Mammograms are typically suggested for women every year after they turn 40. It is recommended that women who are at a higher risk for breast cancer should talk with their doctors about an appropriate screening plan for them.[58][59]
- Magnetic Resonance Imaging (MRI): A MRI may provide a more detailed look at the breast tissue compared to a mammogram or ultrasound. MRIs are move expensive but may show a lump that the other test did not pick up on previously.[58][59]Women who are at higher risk are recommended to not only receive yearly mammograms but should also receive a yearly MRI. Ultimately though women should discuss the appropriate screening process with their doctor.[61]
- Biopsy: A biopsy is a procedure that is performed to detect whether the breast tissue that has been removed is cancerous or not. This test gives a definite answer to whether cancer is present. A or not biopsy is suggested if there is an area within the breast that is questionable for cancer.[58][59] Hormone Receptor Tests If someone is diagnosed with breast cancer, hormone receptor tests can be used to help develop treatment options. If the cancerous tissue is positive for hormone receptors (estrogen and/or progesterone) then hormone therapy is a recommended form of treatment.[58][59]
- HER2/neu Test: HER2 is the human epidermal growth factor receptor-2, which is a protein that can sometimes be found on cancer cells. The cancer cells that contain the HER2/neu protein tend to be more aggressive and may have a less favourable prognosis. If this is the case, then a targeted approach to that specific area will be used as a treatment option.[58][59][62]
Factors that May Reduce Breast Cancer Risk[edit | edit source]
Systemic Involvement[edit | edit source]
Breast cancer that has metastasized can be manifested in several ways[59][46].
- Bone: is the most frequent site of metastasis in both men and women and symptoms can include back hip or shoulder pain, and/or pain with weight-bearing.
- Central Nervous System: is another frequent site for metastasizes of breast cancer, especially at the thoracic levels of the spinal cord. Signs and symptoms that are associated with neurologic involvement include unilateral upper extremity numbness and tingling (cervical/thoracic), leg weakness or paresis (lumbar), or bowel and bladder symptoms (sacral). Other common sites of metastases are lymph nodes, lung, brain, and liver, as well as the remaining breast tissue. Neurologic involvement can also be manifested in a paraneoplastic syndrome, which is a term used to describe associated signs and symptoms at a site that is distant from the tumour and/or metastasis.
- Paraneoplastic syndromes often present in ways that seem uncorrelated with cancer and may mimic disorders of the endocrine, metabolic, hematologic, or neuromuscular systems. Clinical signs and symptoms can accompany a relatively limited increase in the size of cancer and therefore may provide early clues to the presence of cancer. Stiff-man syndrome is an example of a paraneoplastic syndrome that affects women with breast cancer and is characterized by progressive symptoms of neuropathy (nerve damage) or myelopathy (spinal cord damage). Increased muscle tone and rigidity in the spine and lower extremities (especially the ankle dorsiflexors) are commonly experienced.
Musculoskeletal and integumentary involvement, as they relate to breast cancer prior to treatment, have been previously discussed in the section on Characteristics/Clinical Presentation.
Differential Diagnosis[edit | edit source]
There are several other conditions that may be associated with breast pain other than breast cancer itself. Mastodynia, mastitis, benign tumors/cysts, and Paget's disease are some examples of conditions that may cause a patient to present with breast pain resembling breast cancer.
- Mastodynia: is an irritation of the upper dorsal intercostal nerve. This type of breast pain may be associated with ovulatory cycles.
- Mastitis: this occurs in lactating women that is an inflammatory condition. The breast may become red, swollen, painful, and/or warm. This is a result of the mammary duct becoming obstructed and clogged.[46]
- Benign Tumors/Cysts: These include fibroadenomas, cysts, and calcifications within the breast. When lumps within the breast are unchanged and have been present for many years this is often times a benign and hormonally induced. Other benign lumps include, papillomas, fat necrosis, and mammary duct ectasia. The patient would need to be referred to their doctor to differentiate these conditions.[46]
- Paget's Disease: This is a disease of the breast not to be confused with Paget's disease of the bone. It is a rare condition of ductal carcinoma which arises from the ducts near the nipple. Symptoms may include, redness, itching, and flaking of the nipple.[46]
Management[edit | edit source]
Treatment for breast cancer depends on the severity and stage of the disease that the patient is in at the time. In many cases, there is a typical sequence that is followed.
Surgery[edit | edit source]
Surgery is usually the first step in the treatment of breast cancer. The goal of surgery is to remove the cancerous tumor by either removing the entire breast (mastectomy) or removing only the lump and surrounding tissue (lumpectomy).
Below is a brief outline of the common procedures. Selection criteria for surgery:
- The size of the tumour present and the location
- Whether or not the cancer cells have spread
- Breast size
- Personal wishes of the patient
Other forms of surgery such as breast reconstruction and lymph node removal can be done when necessary. After the surgery is performed the patient may receive chemotherapy and/or radiation therapy. A retrospective review shows that in comparison to subcutaneous mastectomy with implant procedure, the partial mastectomy with mini latissimus dorsi flap procedure produces significantly superior body image and cosmetic outcomes. It can also be a treatment of choice in selected patients with small breasts and a high tumor/breast ratio[64].
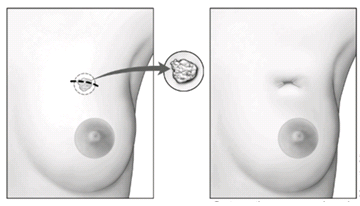
Excisional Breast Biopsy (Lumpectomy)[edit | edit source]
- A lumpectomy, otherwise known as a wide local excision, tends to be used in early stage cancer or when the tumour is of small size.
- The surgeon will remove the cancer cells, as well as some of the healthy tissue which surrounds the affected area. *This can be done with general or local anaesthetic, with the aim of determining the mass of the tumour and to rule out any carcinoma.
- Due to the conserving nature of this procedure, it is one that is favoured by the breast cancer population, and has even been shown to have no significant difference in survival rate when compared with a mastectomy [65].
- The excised tumour is examined further for cancerous cells, if which are found around the edges, may lead to further surgery or mastectomy
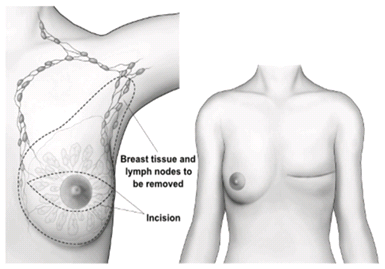
Subcutaneous Mastectomy (Complete Mastectomy)[edit | edit source]
- This procedure results in complete removal of the breast tissue only (>99% of breast tissue), where a carcinoma is in situ.
- When the size of the tumour is large in proportion to the rest of the breast tissue, or is wide spread, a mastectomy is performed.
- As previously mentioned, a mastectomy may be performed if a lumpectomy is unsuccessful in the removal of all cancer cells.
- It may also be performed if cancer cells were to recur following other treatments such as radiotherapy. *Occasionally, complete removal of the pectoralis muscle group can occur if cancer cells were to spread into the musculature.
Modified Radical Mastectomy (MRM)[edit | edit source]
MRM indicated where:
- Tumour location, size, and presence of multiple cancer cells in the breast tissue
- Where radiotherapy is contraindicated.
- Dissection and removal of the lymph nodes within the axilla occurs. The likeliness of morbidity in the arm [66] is increased and can even lead to permanent lymphedema depending on the amount of lymph nodes removed.
Dissection sites:
- Lateral edge of the sternum, clavicle, within latissimus dorsi, rectus sheath
- Breast tissue’s usually dissected from the pectoralis muscle; occasionally compromised.
Strain injuries to the brachial plexus are common, but usually resolve over time. Injuries to the long thoracic nerve are quite common following this particular surgery. Loss of range of motion (ROM) in the glenohumeral joint (GHJ) is quite common following this procedure, physiotherapy has been shown to manage this [66].
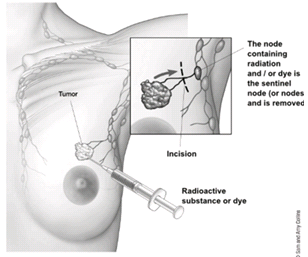
Sentinel Lymph Node Biopsy[edit | edit source]
- Radioactive dye is injected into the breast tissue as a further diagnostic measure to evaluate whether the cancer cells have entered the lymph nodes or not.
- Once identified, the lymph nodes containing the cancer cells, i.e. the sentinel lymph nodes, are then removed surgically.
Chemotherapy[edit | edit source]
Chemotherapy is used to destroy the remaining cancer cells that may be left within the body. This form of treatment is applied to the whole body through the bloodstream. Chemotherapy can be used with all stages of breast cancer but is especially recommended for those patients in which cancer has spread.
- Chemotherapy is a systemic therapy with the aim of killing the dividing cancer cells present in the tissue through the use of a single of various different drugs.
- Adjuvant chemotherapy prolongs disease free and overall survival in patients with early breast cancer [67], and is primarily used in the earlier stages of the disease.
- Used if cancer cells have spread from the original site, or if there is risk that it will spread.
- It can be used as a curative therapy, as well as in conjunction with radiotherapy [68], or can be used to help relieve some symptoms present in the later stages of cancer.
- Treatment processes usually last up to 6 months.
- Chemotherapy may involve the use of a single drug, or a combination of different drugs. A full list of drugs used in cancer treatment, along with their side effects, can be located here.
The benefit of chemotherapy is evident, in contrast, cardiac side effects are also quite common amongst patients treated with chemotherapy. Depending on the drug used, various cardiac side effects can develop, such as:
- Myocardial ischemia
- Left ventricle dysfunction Although these issues cannot be directly influenced through physiotherapy, knowledge of the potential presence of these conditions is vital [69]. Chemotherapy-induced cardiac toxicity is an ever-growing problem, with research stating patients receiving chemotherapy are placed at Stage A heart failure, increasing the risk of cardiac dysfunction in the future [70], with the chance of heart failure possibly being higher than the chance of cancer recurrence [71]
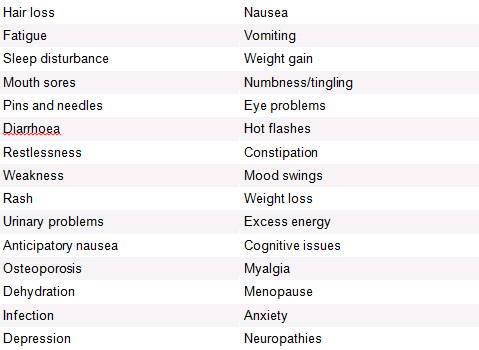
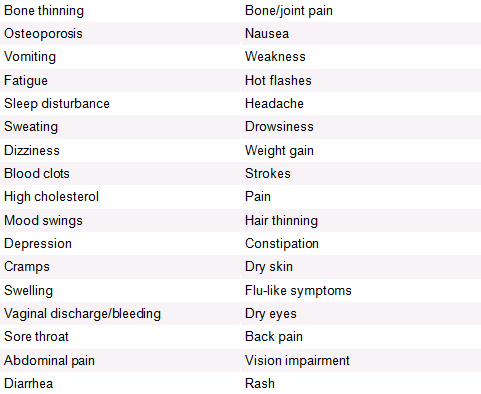
Chemotherapy Side Effects[edit | edit source]
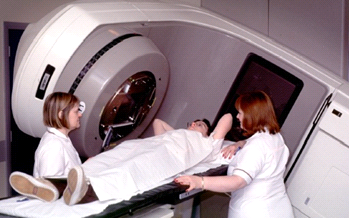
Radiation Therapy[edit | edit source]
Radiation therapy is typically used for early stages (can be used in all stages) of breast cancer following a lumpectomy. This form of treatment targets a more specific area unlike chemotherapy. Radiation therapy may also be used following chemotherapy.
- Almost half of cancer patients will use radiotherapy over the course of their cancer treatment.
- Designed to eliminate cancer cells through the application of high-radiation energy to the affected site. One of the main causes of arm pain in cancer patients [72]
- Risk factor for breast cancer related lymphedema.
- It is often used in with and separately to chemotherapy
- Radiotherapy can be used as a neoadjuvant treatment, or as an adjuvant treatment.
- The main muscles in the line of radiotherapy are the pectoralis muscles, latissimus dorsi, and serratus anterior. Although radiotherapy is a successful tool in the treatment of cancer, there are quite a few side effects, which may last days to years. Skin irritation, for example, is one of the most common side effects [73], with patients experiencing erythema, skin peeling, and even necrosis [74]. As previously mentioned, the direct contact radiotherapy has on the muscles pectoralis major, latissimus dorsi, and serratus anterior, can lead to damage of these healthy tissues, causing pain and inflammation. As in relation to the post-surgical posture which was previously mentioned, patients can tend to adapt a protective posture due to increased sensitivity and fear of movement, again predisposing to contractures and a decreased ROM [75].
Radiotherapy Side Effects[edit | edit source]
Radiation-Induced Fibrosis (RIF)[edit | edit source]
RIF is common following high dose radiotherapy which causes secondary damage to the normal tissues which surround the cancer sites. 45% of patients report RIF related breast pain, which in turn negatively effects the patients’ quality of life [76].
Hormonal Therapy[edit | edit source]
Once the following treatment options have been completed hormonal therapy may be advised. Hormonal therapy works by decreasing estrogen amounts and blocking its action on the breast cancer cells. The doctor and the patient will discuss each specific case and decide on the best treatment options.[58][61]
It will only work in oestrogen receptor positive cancers. Oestrogen receptor positive cancer cells contain a hormone receptor which enables the binding of oestrogen onto the cancer cells, enabling further growth of the cancer. These same receptors are present on normal and healthy breast tissue cells. Hormone therapy aims to:
- To limit growth of the spreading hormone positive cancer cells
- To prevent recurrence
- To decrease the risk of developing hormone positive cancers in the future Aromatase inhibitors are drugs used in hormone therapy. Aromatase is involved in the biosynthesis of oestrogen from androgens located in various tissues throughout the body, primarily adipose tissue in menopausal women. Aromatase inhibitors also lead to a loss of bone density, further predisposing the breast cancer patient to osteoporosis. Therefore, the role of the physiotherapist in hormone therapy should be advice and education, accompanied with cardiovascular and light resistance training with the aim of decreasing chance of obesity and lowering the stress on the potentially weakened bones [80].
Hormone Therapy Side Effects[edit | edit source]
Like with chemotherapy, there are many different medications used in hormone therapy, each of which have different side effects.
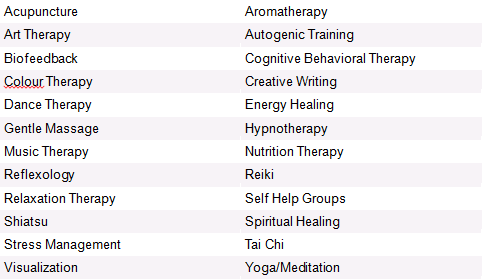
Complimentary Therapies[edit | edit source]
There are many therapies which could possibly be discussed with a breast cancer patient, with the aim of helping them cope through their rehabilitation, any negative symptoms, and to promote their general well-being.
Medications[edit | edit source]
Medications for the treatment of breast cancer most often include chemotherapy drugs and hormone replacement drugs. Chemotherapy medications are many times used in combinations of two or three at a time. Two common groups include anthracyclines and taxanes. Anthracyclines such as, Epirubicin and Doxorubicin, are similar to antibiotics that destroy the cancer cells’ genetic material. Taxanes such as Paclitaxel and Docetaxel, on the other hand, interfere with how the division of the cancer cells.[61] Paclitaxel and Docetaxel are both categorized as plant alkaloid anticancer drugs. Each are given intravenously and used mostly to treat solid tumors involving breast and ovarian cancers. Toxicities are common in cancer treatment and each drug is not alike. The acute toxicity of Docetaxel is hypersensitivity and a rash and delayed toxicity results in neurotoxicity, fluid retention, neutropenia, alopecia, and bone marrow depression.[62] Hormone therapies such as the drug Tamoxifen stop the growth, spread, or recurrence of ER-positive tumors by preventing estrogen from reaching the tumors. Tamoxifen is a mixed estrogen antagonist and agonist that blocks the estrogen activation in the breast and decreases growth factors in the breast tissue. The side-effects are similar to postmenopausal symptoms: hot flashes, nausea, irregular menses, vaginal bleeding and weight gain, as well as slightly increasing a woman's risk for endometrial cancer.[81] Tamoxifen is the most common drug used for premenopausal women to help prevent the recurrence of breast cancer and another drug, Toremifene is the newer estrogen receptor antagonist that is being used in cases of advanced breast cancer.[59][62] Tamoxifen also appears to have a preventive effect in women with a high risk of breast cancer and has now been approved as a chemopreventive agent in this population. [62]
Drug Warning: Cancer patients who receive the targeted therapy bevacizumab (Avastin) in combination with chemotherapy are at increased risk of serious side effects that may lead to death, according to a meta-analysis of 16 clinical trials that was conducted by researchers at Stony Brook University School of Medicine in New York.[82] Fatal events, most commonly hemorrhaging, only occurred in 2.5% of the participants receiving the Avastin treatment compared to those not receiving (1.7% of fatality). The approximated 50% increase in risk occurred in patients also prescribed platinum or taxane chemotherapy agents such as carboplatin and paclitaxel. The results were published in a meta-analysis by Rapura and colleagues (2011).[82]
Newly FDA Approved Drug: Treatment with eribulin (Halaven™) improved overall survival in women with metastatic breast cancer whose disease progressed despite multiple rounds of prior chemotherapy, according to the results of a phase III clinical trial called EMBRACE. Based on these findings, the FDA approved eribulin last November for women with metastatic disease who have already undergone at least two previous chemotherapy regimens. Eribulin is a laboratory-made form of halichondrin B, a substance derived from a sea sponge. It targets the protein tubulin cells (building blocks of microtubules (narrow, hollow tubes inside a cell), involved in cell division and cell movement), although it binds to tubulin in a different way, interfering with cancer cell division and growth. Women receiving eribulin lived 2.5 months longer than women treated with their physician’s drug of choice and had equal side effects of neutropenia, leucopenia, and peripheral neuropathy.[83]
Physical Therapy Management[edit | edit source]
After treatment for breast cancer, women may experience any of the following impairments that can be addressed by a physical therapist:
- Decreased strength of the upper extremity
- Decreased shoulder mobility
- Scar tightness (breast and/or axilla)
- Upper extremity ache
- Lymphedema of the upper extremity
- Neuropathic pain
- Musculoskeletal pain (breast, axilla, and/or neck-shoulder)
- Chronic pain
Musculoskeletal Physiotherapy Interventions Post Surgery:
A physiotherapists treatment plan should include:
- Motion exercises to improve tissue extensibility and facilitate normal movement patterns.
- Myofascial release for enhancing mobility and enhancing tissue extensibility. [84] [85] [86] [87]
Several forms of manual therapy can be carried out by a physical therapist to address certain impairments. They include the following:
- Joint mobilization techniques
- Soft tissue release techniques
- Neurodynamic techniques
Mobility exercises[edit | edit source]
The two most common complications that are evident in breast cancer patients are restricted arm motion and lymphedema. These result in the occurrence of pain at the place of operation and muscle spasms are quite common. It is very important that early rehabilitation is implemented to promote functional movement to the patient’s previous level of activity. Subsequent to operation arm mobilisations are implemented first or second day post-op. Mobilisations are performed using joint rotations to tolerance but abduction and flexion are limited to 40°. At day 4 post-op flexion and abduction are gradually increased to 45°, this can be increased furthermore by 10-15° per day dependent on the patient’s pain tolerance. The technique performed by holding the patients arm in 45° flexion or abduction until the drains are removed. Secondary lymphedema is a common occurrence in the breast cancer population following surgery and has a long term negative effect on patient quality of life. Risk factors that are prevalent in the breast cancer population are age, obesity and the growing survival rate. The growing risk factors make secondary lymphedema a challenging complication in the breast cancer population.
Physiotherapy Interventions for Lymphedema[edit | edit source]
Complex decongestive physiotherapy for lymphedema[edit | edit source]
This is a treatment that incorporates skin hygiene, manual lymph drainage, bandaging, exercises and support garments. Manual lymphoedema drainage is a massage technique that involves the skin surface only. This follows the anatomical lymphatic pathway. Generally the manual lymph drainage technique will begin centrally in the neck and trunk to alleviate any lymphedema in the main lymphatic pathway, so that drainage in the arm is facilitated. Complex decongestive physiotherapy has been suggested as the primary treatment for breast cancer patients. This treatment includes skin care, exercises, compression and manual lymphedema treatment. Complex decongestive physiotherapy has shown to be an effective method for the treatment of lymphedema when standard elastic compression has been unsuccessful. One study showed consistent results of the reduction in volume of the effected extremity in 95% of 400 patients. A follow-up showed that these therapeutic results were maintained at 3 years.
Elevation[edit | edit source]
Elevation has been described as a non-effective treatment when performed solely in breast cancer treatment for arm related oedema. Elevation is commonly used together with other treatments to provide the most effective treatment. Most commonly in breast cancer treatment a multidisciplinary approach is taken where the patient will undergo massage and exercise. A specific technique of massage is commonly implemented named manual lymphatic drainage. Manual lymphatic drainage is a type of massage used to mobilise oedema fluid from distal to proximal areas and from areas of stasis to healthy lymphatics.
Compression[edit | edit source]
One form of lymphedema treatment which has proved most effective in the treatment of breast cancer patients is the use of standard elastic compression garments. Studies have shown significant improvements using simple elastic compression treatment for lymphedema, where 34% of patients showed a substantial reduction in arm oedema at 2 months and 39% of patients at 6 months. This conclusion was also seen in patients older than 65 years old [88].
Physiotherapy to prevent secondary lymphedema arising[edit | edit source]
- Stretching for muscles of the shoulder: Levator scapulae; upper trapezius; pectoralis major and the medial and lateral rotators of the shoulder
- Progressive active and active assisted shoulder exercises.
- Functional exercise activities.
- Proprioceptive neuromuscular facilitation exercises.
Also see page on Lymphedema for treatment of BCRL.
Physical Activity[edit | edit source]
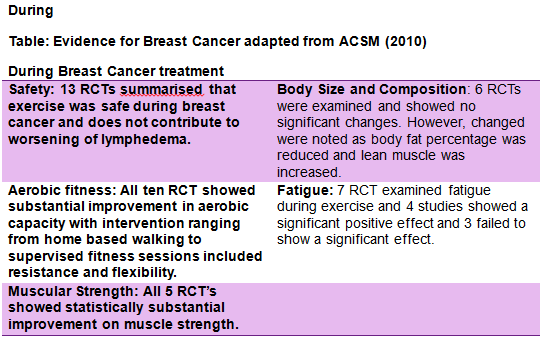
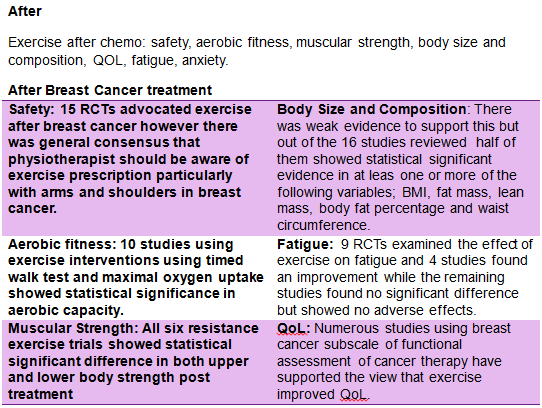
Exercise is increasingly being implemented as a therapeutic tool in patients with breast cancer [89]. In recent times it has become clear that exercise has a central role to play in controlling and preventing chronic illness. However, statistically breast cancer survivors have a very low compliant rate and despite the renowned benefits of exercise, 22% Canadian cancer survivors report being inactive [90]. Physical exercise has shown to be a suitable adjunct therapy to battle long term chronic conditions and has been successful in reducing mortality and improving overall quality of life. There is substantial evidence to support the benefits of exercise in breast cancer in both during and after chemotherapy. Research has shown that physical activity and exercise is effective in improving QoL, cardiorespiratory fitness, physical functioning in breast cancer patients and survivors [91]. These studies where compared with similar studies and analysed in separate components such as safety, aerobic fitness, muscular strength, body size and composition, QOL, fatigue, anxiety. Physical exercise has shown to be a suitable adjunct therapy to battle long term chronic conditions and has been successful in reducing mortality and improving overall quality of life. A review of the current literature by Corneya [89] carried out an overview of research on the effect of exercise on cancer. Twelve studies met the inclusion criteria and showed statistically significantly beneficial results in the effects of exercise during breast cancer. More so, the studies demonstrated benefits exercise capacity, body weight and overall quality of life. The ACSM [91] discussed guidelines for cancer survivors and analysed studies during and after breast cancer separately. The ACSM[91] compared studies during breast cancer using the following headings; safety, aerobic fitness, muscular strength, body size and composition, QOL, fatigue, anxiety.
Precautions[edit | edit source]
There is a general assumption that upper limb exercises could be harmful to patients who had axillary nodes removed or radiation the axilla, however the latest research does not support this view.
When performing exercise for post surgical populations the SEWS chart should be monitored regularly for early warning signs. If the patient is feeling fatigued or anaemic exercise should be delayed.
Exercise Prescription[edit | edit source]
The ACSM guideline suggests that an overall volume of 150 mins of moderate-intensity exercise or 75 min of vigorous intensity exercise. Resistance exercise should be performed 2-3 times weekly with exercises including the major muscle groups. However, with patients undergoing cancer treatment the key is to avoid inactivity and the patient should remain as active at their ability and condition allows them.
FITT Guidelines[edit | edit source]
There is a huge gap at present in the research governing exercises prescription and its translation to the real world and community settings. As discussed earlier exercise compliance post cancer is very low and a qualitative study by Miedema [92] stated numerous factors for this such as lack of availability of services, travel issues, cost and personal reasons as family responsibilities and fatigue. Physiotherapist should be aware of the barriers to exercise compliance in this specific population (See #Barriers ).
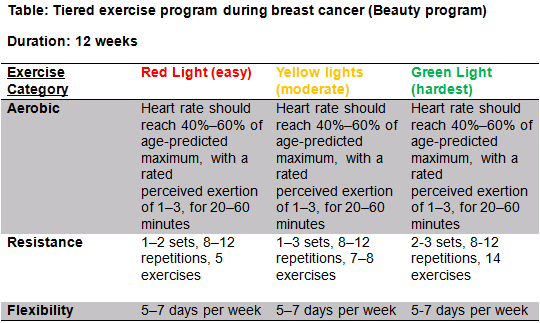
The beauty program aims to counteract key concerns associated with breast cancer patients such as fatigue, reduced QoL, social anxiety and physical conditioning. Considering there is huge physiological benefits as well major psychological benefits it is important that the physiotherapist promotes the benefits of exercise immediately post-surgery and ensures that the exercise program is assessable at home or in the community and is specific to the individual. All exercise programs should be designed with F.I.T.T principles during and after breast cancer. A recent study by Leach [93] designed an exercise program called BEAUTY (The breast cancer patient engaging in activity and undergoing treatment program).The beauty program aims to bridge the gap between the body of evidence supporting the role of exercise in breast cancer and implementing this in a real world setting.
BEAUTY:
Patients were assessed by exercise physiologist before undergoing exercise treatment and underwent a variety of tests to get measure of baseline fitness and also filled out questionnaires relating to exercise and medical history, fatigue, QoL and depressive symptoms.
FITT Principle After Breast Cancer[edit | edit source]
- Warm up: 5-10 minutes to raise heart rate
- Aerobic Exercise: Frequency:
- 3 x 5 times per week **Intensity: 50-70% of max. heart rate
- Type: walking cycling aerobic activity
- Time: 30 minutes maintaining as a long term routine
- Resistance Training: Frequency:
- 2/3 times a week
- Intensity: 12/15 reps of 60 % of 1RM
- Type: Supervised resistance program of major muscle groups
- Time: 6 weeks
Karki and colleagues (2005)[94] researched the effects that impairments have on activity limitations and participation restrictions in 96 women at 6- and 12-month follow-ups after an operation for breast cancer. The severity of the impairments were measured using a modified Visual Analogue Scale (VAS).[94] Activity limitations and sleep function were measured using the Behavioural Rate Scale for Patients with Breast Cancer.[94] Results showed increased impairment severity with upper extremity lifting and carrying, as well as sleep disturbances, regardless of the type of operation.[94] While the prevalence decreased at the 12-month follow-up, changes were not statistically significant.[94] Results also show that women experience constant restrictions at home, work, and during leisurely activities, which may also have a negative impact on their social health.[94]
Aerobic exercise, such as walking, cycling, or swimming, has been shown to decrease cancer-related fatigue,[95][96][97] improve quality of life,[98][99] reduce cognitive impairments associated with various cancer therapies,[100] improve cardiovascular outcomes,[101] and improve sleep dysfunction.[102] Research suggests that treadmill exercises provide cardioprotective effects on the Doxorubicin-induced cardiotoxicity.[103]
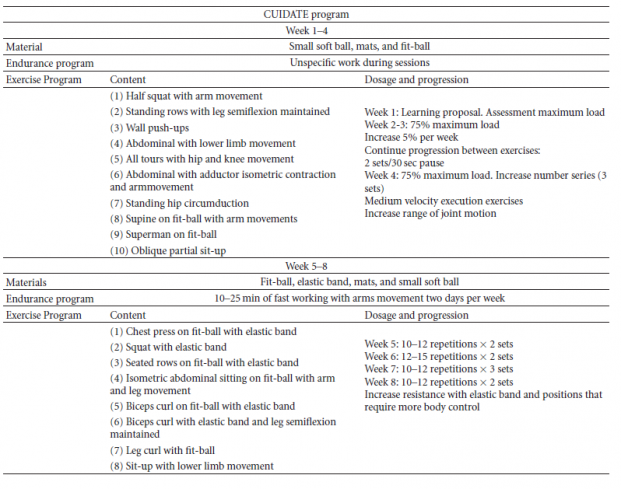
Cantarero-Villanueva and colleagues (2011)[104] carried out an 8-week multimodal physiotherapy program, which was comprised of aerobic exercises, core stability exercises, and some recovery with stretching and myofascial release techniques.[104] The researchers found statistically significant improvements in strength, suggesting that core stability exercises should not be ignored in women who have undergone treatment for breast cancer.[104] In fact, chemotherapy has been found to lead to even more muscle atrophy.[105] The protocol that Cantarero-Villanueva and colleagues (2011)[104] followed can be seen in the table below.
Other muscle groups that should be targeted include the rotator cuff, serratus anterior, trapezius, rhomboids, biceps, and pectoralis muscles.[106] Exercises can begin with an elastic bands and be performed 2x/week for 2 sets of 10-15 repetitions.[107] However, it is also important to observe the individual's scapulohumeral rhythm and supplement the exercise program with neuromuscular facilitation exercises if it needs to be corrected.[108][109] This study aimed to analyze the effects of types of surgery and hormone therapy on muscle strength in breast cancer survivors suggested that breast cancer survivors should be encouraged to perform supervised strength training programs for different muscle groups to improve strength[110].
Barriers, Motivators and Myths and Physical Activity[edit | edit source]
- Breast cancer patients report more barriers to exercise than other cancer groups [111].
- The barriers are having a clear impact on activity participation.
- Only 30-47% of cancer survivors are undertaking the physical activity guidelines of 30 minutes of moderate intensity activity per day [112]
- Understanding the barriers can help physiotherapists in making services more accessible to breast cancer survivors and encourage participation.
Myths[edit | edit source]
Physiotherapists have an important role to play as educators in dispelling the myths and presenting patients with evidence to reassure them that exercise will not be harmful. Survivors often report concerns about the safety and benefits of exercise for their condition, however it is important to note that exercise both during and after cancer treatment has no known significantly negative effects [89] [113] [114].
Barriers[edit | edit source]
The main themes identified from interviews with survivors are physical, environmental and psychosocial [115]. Top 10 patient reported barriers to exercise [116]
- Concerns about their illness and other health problems
- Joint stiffness
- Fatigue
- Pain
- Lack of motivation
- Weather
- Lack of facilities
- Weakness
- Lack of interest
- Fear
Psychological Barriers[edit | edit source]
Psychological barriers identified from a large qualitative study by Hefferon et al [117] included:
- Lack of motivation
- Fear of injury
- Fear of lymphodema
- Dislike of gym environment
- Lack of privacy
“I just couldn’t … I had no energy to do anything. I could hardly drag one leg after the other...” - Anon [117]
"I had my breast off, I had to go through a year later for another operation … you’re all kind of sore and tense [...] plus the fact that the chemotherapy affected the nerve in my feet, so I didn’t have the strength ... I didn’t have the feeling in my feet.” - Anon (Hefferon et al. 2013)
Low mood and depression are commonly reported by patients and understandably make motivation to exercise extremely difficult.
“I feel sad. Even when I wanted to go out.…With other kind of physical sickness it’s ok but with cancer… so difficult to get myself out of this…” -Anon [118]
Physical Barriers[edit | edit source]
The following may contribute to negative feelings towards exercise participation [119]:
- Cancer treatment side-effects including weakness, loss of shoulder movement, musculoskeletal pain, neuropathy, weight gain and breast cancer related lymphoedema
- Post surgery complications e.g numbness, shoulder stiffness, lymphodema [120]
- Cancer or age related aches and pains
- More than half of patients report fatigue and 68% of participants in one study reported not having been told how to manage it [121]
Breast Cancer Related Lymphodema (BCRL)[edit | edit source]
BCRL manifests as chronic oedema of the upper limb and trunk in response to damaged axilliary lymph nodes as a result of surgery. As a result patients experience pain, a heavy feeling in the arm, psychological distress and self image concerns as well as increased risk of infection [122] [123]. Occurrence differs for populations of survivors, with prevalence being reported as anything between 2 and 83% [124]. In the past it was common belief that exercise could exacerbate BCRL and as such women were discouraged from exercises such as weight training [125], [124]. Research inspired by McKenzie et al's [126] “Abreast in a Boat – a race against breast cancer” has since refuted this, leading to a new culture of exercise promotion in breast cancer survivors.
Dragon Boat:
The subject is still a source of confusion and concern for many survivors. At present it cannot be said for certain that exercise will not increase occurrence of BCRL, however recent research does suggest that exercise does not cause clinically harmful effects related to BCRL [127],[124]. Encouragingly, studies on weight training and BCLR found no significant link between exercise and increased oedema when the exercises were closely supervised and progressed gradually [128] [129]. Furthermore, the implementation of early physiotherapy may lower or eliminate the occurrence of secondary lymphoedema in breast cancer patients who have undergone surgery.
Psychosocial Barriers[edit | edit source]
Patients may have Psychosocial barriers to exercise such as time constraints, issues with return to work, family commitments, as well as the facilities and environments available for exercise participation. Cancer incidence is high in women aged between 40-50. Many in this age group are active, working mothers with families and many responsibilities who devote much of their time to family and child care. We must also consider the time demands that come with multiple hospital appointments during and after treatment. As therapists we must therefore consider where and when the patient can exercise, what alternatives and comprises can be made as an alternative to gym based exercise.
Barriers During Versus After Treatment[edit | edit source]
Recently diagnosed women expressed more negative feelings towards physical activity than those after treatment, and may be less aware of the benefits of activity. Those who were currently undergoing treatment state that the need to conserve energy and fear of infection were of great concern to them when deciding to exercise. [130].
Motivators[edit | edit source]
Social support is commonly reported as a motivator for survivors. Group programs are said to provide a supportive, safe environment [131], which therapists should keep in mind when offering exercise advice.
“I think it is good to find a group of people and do together …because being alone is very lonely and harder to sustain the physical activity.’-Anon [131]
Common motivators reported include weight loss, health benefits, increased energy, body image and social support [132]. One patient stated the importance of exercise facilitators being understanding and caring.
“...the instructor that I had was very caring. I couldn’t keep up through the whole class so she would come up to me and say: ‘Are you doing ok?’ I think that helped me. It made me feel, not that people felt sorry for me, but were concerned that I was okay.” Anon [132]
Top 10 patient reported motivators for exercise [133]
- Fun
- Variety of exercises
- Gradual progression
- Flexible
- Personal goal setting
- Good music
- Individually tailored
- Feedback given
- Oncologist approval
- GP approval
Psychological Issues[edit | edit source]
Breast cancer diagnosis is a traumatic experience affecting not only the patient but all involved in the patient’s life. Cancer patients go through an emotional journey that affects their psychological wellbeing and mental health. It is therefore important that alongside the physiological treatment, care is taken of the patient’s emotional wellbeing to protect their long-term quality of life. The psychological issues surrounding the breast cancer patients, their carers and health workers, should be addressed at an early stage by ensuring that support and guidance is available to patients and others in need.
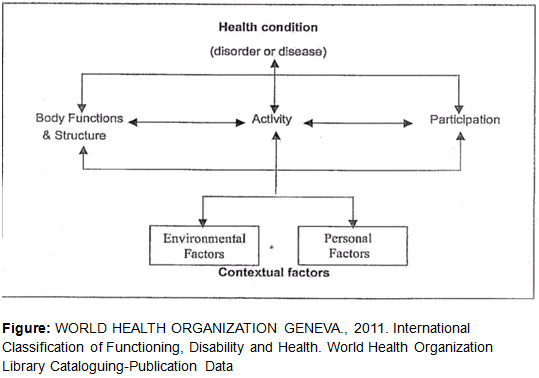
Biopsychosocial Model
The World Health Organization defines the biopsychosocial model as:
“attempts to achieve a synthesis, in order to provide a coherent view of different perspectives of health from a biological, individual and social perspective” [134]
The goal of the ICF classification is to provide a unified language and framework to describe health and health-related states. The ICF has two parts:
- Functioning and Disability - includes body function and structures, and activities and participation. #Contextual Factors - includes environmental factors, as well as personal factors. These represent the individual’s background and lifestyle. The environmental factors are the physical, social and attitudinal environment in which the person lives. They are external factors and can either be positive or negative influences on the individual’s performance as a member of society, on the individual’s ability to complete tasks, or on the individual’s body function and structure. (see #Barriers.2C_motivators_and_myths_behind_physical_activity_in_breast_cancer_survivors )
Environmental Factors[edit | edit source]
These include the immediate environment e.g home, workplace or school. Other individuals who whom they have direct contact are also included. The second focus is the way the environmental factors relate to functioning and disability. Different environments may produce different impacts on the same individual with a given health condition. An environment with barriers will restrict an individual’s performance in society, while an environment that is more facilitating may enhance their performance. (see #Barriers.2C_motivators_and_myths_behind_physical_activity_in_breast_cancer_survivors )
Personal Factors[edit | edit source]
This includes important aspects of the individual not directly related to their health status. They include things such as gender, race, age, other health conditions, fitness, lifestyle, habits, upbringing, coping styles, social background, education, profession, past and current experience, overall behavior pattern and character style, individual psychological assets and other characteristics, all or which may play a role in any level of disability. The ICF is based on the integration of the functioning and disability model with contextual factors. This is in an attempt to capture the integration of various perspectives of functioning. Thus, it attempts to achieve a synthesis, in order to provide a coherent view of different perspectives of health from a biological, individual and social perspective. As such the biopsychosocial model provides a framework to assist healthcare professionals in “expanding their repertoire of anxiety management strategies and empowering patients to learn self-healing modalities that interrupt major anxiety pathways, thereby contributing to secondary prevention.” [135] Working through the biopsychosocial model of health, it is clear that in our role as physiotherapists we need to address more than just patient’s physical needs. All the needs and concerns need to be treated.
The advancement of science and medical technology has brought a decrease in physical deformity from today’s surgical treatments. said, contemporary breast cancer treatments are becoming more complex and require a longer period of time. This is due to an increase in the available information and due to more opportunities of information and choices given to patients detailing their cancer diagnosis and prognosis and sharing the decision-making process regarding clinical intervention and treatment.
Psychological Aspects[edit | edit source]
Amongst the psychological aspects impacting on the physiological system are depression, self-esteem, fear of recurrence, changes in body image and sexuality, as well as physical toxicities that result from adjuvant therapy. A research carried out by Ganz, P.A. (2008) addresses the importance of identifying the psychological and social concerns of breast cancer patients in the clinical setting, and it assists patients in obtaining appropriate psychosocial services. Further information from this paper can be obtained by following this link. Robotin M. et al [136] discuss the impact that the psychological concerns have on cancer survivors and state that:
“other issues such as difficulties in decision-making and social isolation may emerge months or years after treatment has finished”
This implies the nature of the survivorship – a dynamic experience which changes over time – and the importance of the life style and social support to cancer survivors. Despite recent advances in the treatment of cancer, people have a universal dread of cancer and in most cases the disease remains highly stigmatised.
Strategies for Dealing With Psychological Issues[edit | edit source]
The following are some of the key strategies we should take into account when addressing the psychological problems that may arise from breast cancer diagnosis:
Patient-Centred verbal communication is key and all staff should seek to develop their skills in this aspect as part of the continuous professional development. All forms of communication should be patient-centred. Each health professional should act pro-actively to develop good working relationships with key relevant players and patients, including MDTs. To address and overcome these psychological issues it is imperative to have effective communication tools that can be used and accessed by all those involved in the patient health journey.
Physiotherapy Long-term Management[edit | edit source]
When exploring and defining strategies for long-term physiotherapy management of patients with breast cancer, barriers, motivators and myths behind physical activity in breast cancer survivors should also be addressed.
The role of a physiotherapist is to promote a healthy life style including physical activity and proper nutrition. As highlighted previously, exercise interventions are being used and incorporated into treatment however much more research is needed to implement it on a higher standers of care level. Continuation of exercise can continue to foster motivation in patients, provide a support group for patients, enable social and psychological wellbeing. Most of all it can improve patients quality of life. It allows patients to have some control over their lives, stability and routine. It allows them to regain themselves and return to being active in a community [137].
Education[edit | edit source]
Education plays a vital role in patient centred care and clinician evidence base practice. There is clear efficacy for the use of education as has been highlighted throughout this resource. Breast Cancer Care developed a resource for exrcise after breast cancer surgery and is readily available here. McMillan Cancer Support also have another useful resource targeted towards marketing activitiy entitled Move More
The ACPOHE state that education of the patient is a key component of the physiotherapists role. ACPOHE recommend promotion of physical activity, independence and self-management as greatly important for successful rehabilitation outcomes. In reference to the biopsychosocial model of health, it is clear that physiotherapists have a duty to address more than just the patient’s physical problems. All patient needs and concerns need to be treated, an issue which was highlighted by Karen Middleton (CSP Chief Executive) in a speech at the Physiotherapy UK 2014 conference which may be viewed here.
“...physios can reverse injury, enable people to live with long-term conditions, integrate health and social care and help people back into work...we’re very good value”
Life After Cancer[edit | edit source]
Life after breast cancer treatment means returning to some familiar things and also making some new choices. The end of treatment does not mark the end of the journey with breast cancer. The patient embarks to adjusting to life as a breast cancer survivor and in many way will have a life that is in some ways very different from the life before. These changes include relationships to eating habits and exercise. How do you fight lingering fatigue? What should you eat to help prevent a breast cancer recurrence? Will you ever have a regular sex life again? These are just a few of the questions that may nag at the patient as they make the transition from breast cancer treatment to breast cancer survival. The patient’s body has been through an enormous assault and recovery is a huge thing – the patient cannot bounce back right away. Two of the more frustrating and troubling side effects women face after treatment are fatigue resulting from chemotherapy and/or the accumulated effects of other treatments, and a phenomenon some women have dubbed "chemobrain" -- mental changes such as memory deficits and the inability to focus.
Patients should be advised to make sure that their family and work colleagues understand that just because treatment is over, that doesn't mean that she is going to be able to jump right back into running the office, coaching, and travelling to conferences a week out of every month. The issue of returning to work shortly after the treatment, has been addressed by many organisations, amongst them cancer societies such as the American Cancer Society, who advise that returning to work may help maintain your sense of who you are and how you fit in. However, the return to work should be graded and the possible options should be explored with the employer, like flexi-time, job sharing, or working from home.
Options like these may help the patient ease her mind and body back into the demands of her job. The Association of Chartered Physiotherapists in Occupational Health and Ergonomics referred to their role as:
“Physiotherapists in Occupational Health use their professional knowledge and skills, together with skills for interaction and decision-making/problem-solving to assess the occupational health needs of the workforce, and to design and deliver personalised advice and interventions that maximise an individual’s performance at work”. (ACPOHE 2013)
Vocational Rehabilitation physiotherapists are involved in the early treatment and timely application of appropriate treatment and advice. This allows patients to remain active, suitably return to work following injury, and remain at work upon return [138]. The physiotherapist should develop personalised interventions for each individual patient and duties include assessment of workers abilities, job and task analysis for each individual patient.
The physiotherapist can assist the patient with her plans to return to work by carrying out assessments on the physical capabilities of the patient in relation to the work place. A work place assessment will also benefit the achievement of this goal. Following the workplace assessment, an adjustment of the duties can be recommended to the patient and the employer. The knowledge of anatomy, kinesiology and ergonomics, together with the agreed work place adjustments, will allow the physiotherapist to focus on the treatment of the disease and prevent injuries when the patient returns to work. This will be done by addressing the physical function of the rest of the body which will help the patient maintain independence and confidence. CSP reports, Work Health and Fitness for Work 2014, highlight the role physiotherapy plays in patient’s physical and mental wellbeing and the effectiveness in reducing costs from the sickness absence through improving function and independence by providing advice to the patients and collaborating with the government and other health agencies to support patients to engage with work and influence employers in the decision making of the tasks allocation and management. Fitness Profits is a CSP leaflet which outlines the role of the physiotherapy and the benefits it would have for the patient.
Breast Cancer Recurrence[edit | edit source]
Patients need to be educated to know their bodies and signs and symptoms so if there is recurrence, patients can identify the signs and address this at an early stage. Clinicians should be educated on the potential breast cancer recurrence and have strategies in place to address a reoccurrence for each individual patient. The clinician are expected to know where to turn to within the MDT to treat any issues effectively. The sings and symptoms for reassurance of breast cancer varies between patients and the specific kind of breast cancer, there for clinicians need to undertake specific learning for their patient groups for these.
“Cancer control requires a multilevel strategy that intervenes at different stages of the disease” [139]
Outcome Measures[edit | edit source]
| Lymphodema
LYMQOL is a validated lymphoedema specific outcome measure for QOL [140]. It consists of 24 questions covering 4 domains (symptoms, body image, mood and function. It is measured by a likert scale from 1-4 Cancer Related Fatigure BFI (brief fatigue inventory). The BFI measures the severity and impact of fatigue in a 24 hr duration. 9 items 0-10 numeric scale. [141] The functional assessment of cancer therapy (FACT-F) FACT-F measures physical fatigue and its consequences over a 7 day period. It is a 13-item uni-dimensional scale assessed on a 5-point scale of 0–4. [142] Shoulder Function Disabilities of the Arms, Shoulder, and Hand (DASH). [143] Psychometric Outcome Measure Hospital Anxiety and Depression Scale (HADS). [144] Quality of Life European Organisation for Research & Treatment of Cancer Breast Cancer – Quality of Life Questionnaire-Core 36 (EORTC QLQ-C36) Developed in 1987 by Aaronson et al. |
Resources[edit | edit source]
You can visit some of the websites listed below for more resources.
Download a PDF on Oncology and Breast Cancer
References[edit | edit source]
- ↑ 1.0 1.1 Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA: a cancer journal for clinicians. 2018 Nov;68(6):394-424.
- ↑ Schmitz KH, Speck RM, Rye SA, DiSipio T, Hayes SC. Prevalence of breast cancer treatment sequelae over 6 years of follow‐up: the Pulling Through Study. Cancer. 2012 Apr 15;118(S8):2217-25.
- ↑ Doyle C, Kushi LH, Byers T, Courneya KS, Demark‐Wahnefried W, Grant B, McTiernan A, Rock CL, Thompson C, Gansler T, Andrews KS. Nutrition and physical activity during and after cancer treatment: an American Cancer Society guide for informed choices. CA: a cancer journal for clinicians. 2006 Nov;56(6):323-53.
- ↑ 4.00 4.01 4.02 4.03 4.04 4.05 4.06 4.07 4.08 4.09 4.10 4.11 4.12 4.13 4.14 4.15 4.16 4.17 4.18 4.19 Canadian Cancer Society. Breast Cancer. Available from: https://www.cancer.ca/en/cancer-information/cancer-type/breast/breast-cancer/?region=on [Accessed 2020 June 23].
- ↑ 5.0 5.1 Spittler CA. Breast reconstruction using tissue expanders: assessing patients' needs utilizing a holistic approach. Plastic surgical nursing. 2008 Jan 1;28(1):27-32.
- ↑ 6.0 6.1 Merkle CJ, Loescher LJ. Biology of cancer. Cancer nursing, principles and practice, 6th edn. Jones and Bartlett, Boston. 2005:3-26.
- ↑ Canadian Cancer Society. Metastatic cancer. Available from: https://www.cancer.ca/en/cancer-information/cancer-type/metastatic-cancer/metastatic-cancer/?region=on [Accessed 2020 June 23].
- ↑ Breastcancer.org. Metastatic Breast Cancer Symptoms and Diagnosis. Available from: https://www.breastcancer.org/symptoms/types/recur_metast/metastic [Accessed 2020 June 23].
- ↑ Learn oncology. The basics of breast cancer (5W's). Available from: https://www.youtube.com/watch?v=uy5uwFeffvA&feature=emb_logo [last accessed 16/7/2015]
- ↑ Langhorne ME, Fulton JS, Otto SE. Oncology nursing. : St. Louis, Mo. : Mosby Elsevier, c2007; 5th ed. / edited by] Martha E. Langhorne, Janet S. Fulton, Shirley E. Otto; 2007.
- ↑ Korde LA, Zujewski JA, Kamin L, Giordano S, Domchek S, Anderson WF, Bartlett JM, Gelmon K, Nahleh Z, Bergh J, Cutuli B. Multidisciplinary meeting on male breast cancer: summary and research recommendations. Journal of Clinical Oncology. 2010 Apr 20;28(12):2114.
- ↑ Cutuli B. Strategies in treating male breast cancer. Expert opinion on pharmacotherapy. 2007 Feb 1;8(2):193-202.
- ↑ Gennari R, Curigliano G, Jereczek-Fossa BA, Zurrida S, Renne G, Intra M, Galimberti V, Luini A, Orecchia R, Viale G, Goldhrisch A. Male breast cancer: a special therapeutic problem. Anything new?. International journal of oncology. 2004 Feb 29;24(3):663-70.
- ↑ Fentiman IS, Fourquet A, Hortobagyi GN. Male breast cancer. The Lancet. 2006 Feb 18;367(9510):595-604.
- ↑ Yoney A, Kucuk A, Unsal M. Male breast cancer: a retrospective analysis. Cancer/Radiothérapie. 2009 Apr 1;13(2):103-7.
- ↑ Weiss JR, Moysich KB, Swede H (2005) Epidemiology of male breast cancer. Cancer Epidemiol Biomarkers Prev 14(1):20–26
- ↑ Burga AM, Fadare O, Lininger RA, Tavassoli FA. Invasive carcinomas of the male breast: a morphologic study of the distribution of histologic subtypes and metastatic patterns in 778 cases. Virchows Archiv. 2006 Nov 1;449(5):507-12.
- ↑ Sanguinetti A, Polistena A, Lucchini R, Monacelli M, Galasse S, Avenia S, Triola R, Bugiantella W, Cirocchi R, Rondelli F, Avenia N. Male breast cancer, clinical presentation, diagnosis and treatment: Twenty years of experience in our Breast Unit. International Journal of Surgery Case Reports. 2016 Jan 1;20:8-11.
- ↑ American Cancer Society. Breast cancer facts & figures. 2019-2020; Available from: https://www.cancer.org/content/dam/cancer-org/research/cancer-facts-and-statistics/breast-cancer-facts-and-figures/breast-cancer-facts-and-figures-2019-2020.pdf last accessed [20/07/2020]
- ↑ 20.0 20.1 Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA: a cancer journal for clinicians. 2018 Nov;68(6):394-424.
- ↑ Tao Z, Shi A, Lu C, Song T, Zhang Z, Zhao J. Breast cancer: epidemiology and etiology. Cell biochemistry and biophysics. 2015 Jun 1;72(2):333-8.
- ↑ Coleman MP, Quaresma M, Berrino F, Lutz JM, De Angelis R, Capocaccia R, Baili P, Rachet B, Gatta G, Hakulinen T, Micheli A. Cancer survival in five continents: a worldwide population-based study (CONCORD). The lancet oncology. 2008 Aug 1;9(8):730-56.
- ↑ Gondos A, Chokunonga E, Brenner H, Parkin DM, Sankila R, Borok MZ, Chirenje ZM, Nyakabau AM, Bassett MT. Cancer survival in a southern African urban population. International Journal of Cancer. 2004 Dec 10;112(5):860-4.
- ↑ Yu XQ, O'Connell DL, Forman D. Comparison of cancer survival in UK and Australia: rates are higher in Australia for three major sites. British journal of cancer. 2004 Nov;91(9):1663-5.
- ↑ Gorey KM, Holowaty EJ, Fehringer G, Laukkanen E, Richter NL, Meyer CM. An international comparison of cancer survival: relatively poor areas of Toronto, Ontario and three US metropolitan areas. Journal of Public Health. 2000 Sep 1;22(3):343-8.
- ↑ Petrek JA, Senie RT, Peters M, Rosen PP. Lymphedema in a cohort of breast carcinoma survivors 20 years after diagnosis. Cancer. 2001 Sep 15;92(6):1368-77.
- ↑ Ezzo J, Manheimer E, McNeely ML, Howell DM, Weiss R, Johansson KI, Bao T, Bily L, Tuppo CM, Williams AF, Karadibak D. Manual lymphatic drainage for lymphedema following breast cancer treatment. Cochrane database of systematic reviews. 2015(5).
- ↑ 28.0 28.1 Siegel RL, Miller KD, Jemal A. Cancer statistics, 2019. CA: a cancer journal for clinicians. 2019 Jan;69(1):7-34.
- ↑ 29.0 29.1 Paley PJ. Screening for the major malignancies affecting women: current guidelines. American journal of obstetrics and gynecology. 2001 Apr 1;184(5):1021-30.
- ↑ Brewer HR, Jones ME, Schoemaker MJ, Ashworth A, Swerdlow AJ. Family history and risk of breast cancer: an analysis accounting for family structure. Breast cancer research and treatment. 2017 Aug 1;165(1):193-200.
- ↑ Brewer HR, Jones ME, Schoemaker MJ, Ashworth A, Swerdlow AJ. Family history and risk of breast cancer: an analysis accounting for family structure. Breast cancer research and treatment. 2017 Aug 1;165(1):193-200.
- ↑ Chen S, Parmigiani G. Meta-analysis of BRCA1 and BRCA2 penetrance. Journal of clinical oncology: official journal of the American Society of Clinical Oncology. 2007 Apr 10;25(11):1329.
- ↑ 33.0 33.1 Fejerman L, Ziv E. Population differences in breast cancer severity. Pharmacogenomics. 2008;9(3):323-333
- ↑ Allen NE, Beral V, Casabonne D, Kan SW, Reeves GK, Brown A, Green J. Moderate alcohol intake and cancer incidence in women. Journal of the National Cancer Institute. 2009 Mar 4;101(5):296-305.
- ↑ Aronson K. Alcohol: a recently identified risk factor for breast cancer. Cmaj. 2003 Apr 29;168(9):1147-8.
- ↑ 36.0 36.1 36.2 36.3 Collaborative Group on Hormonal Factors in Breast Cancer. Alcohol, tobacco and breast cancer–collaborative reanalysis of individual data from 53 epidemiological studies, including 58 515 women with breast cancer and 95 067 women without the disease. British journal of cancer. 2002 Nov 18;87(11):1234.
- ↑ Singletary KW, Gapstur SM. Alcohol and breast cancer: review of epidemiologic and experimental evidence and potential mechanisms. Jama. 2001 Nov 7;286(17):2143-51.
- ↑ Yoo KY, Tajima K, Park SK, Kang D, Kim SU, Hirose K, Takeuchi T, Miura S. Postmenopausal obesity as a breast cancer risk factor according to estrogen and progesterone receptor status (Japan). Cancer letters. 2001 Jun 10;167(1):57-63.
- ↑ 39.0 39.1 Washbrook E. Risk factors and epidemiology of breast cancer. Women's Health Medicine. 2006 Jan 1;3(1):8-14.
- ↑ 40.0 40.1 Terry PD, Miller AB, Rohan TE. Cigarette smoking and breast cancer risk: a long latency period?. International journal of cancer. 2002 Aug 20;100(6):723-8.
- ↑ Sun YS, Zhao Z, Yang ZN, Xu F, Lu HJ, Zhu ZY, Shi W, Jiang J, Yao PP, Zhu HP. Risk factors and preventions of breast cancer. International journal of biological sciences. 2017;13(11):1387.
- ↑ Collaborative Group on Hormonal Factors in Breast Cancer. Breast cancer and hormonal contraceptives: collaborative reanalysis of individual data on 53 297 women with breast cancer and 100 239 women without breast cancer from 54 epidemiological studies. The Lancet. 1996 Jun 22;347(9017):1713-27.
- ↑ Million Women Study Collaborators. Breast cancer and hormone-replacement therapy in the Million Women Study. The Lancet. 2003 Aug 9;362(9382):419-27.
- ↑ Narod SA. Hormone replacement therapy and the risk of breast cancer. Nature reviews Clinical oncology. 2011 Nov;8(11):669-76.
- ↑ Winchester DJ, Chang HR, Graves TA, Bland KI, Winchester DP. A comparative analysis of lobular and ductal carcinoma of the breast: presentation, treatment, and outcomes. Journal of the American College of Surgeons. 1998 Apr 1;186(4):416-22.
- ↑ 46.00 46.01 46.02 46.03 46.04 46.05 46.06 46.07 46.08 46.09 46.10 Goodman C, Snyder T. Differential Diagnosis for Physical Therapists: Screening for Referral. St. Louis, Missouri: Saunders Elsevier, 2007. p784-793.
- ↑ EBAUGH, D., SPINELLI, B. AND SCHMITZ, K.H., 2011. “Shoulder impairments and their association with symptomatic rotator cuff disease in breast cancer survivors”, Medical Hypotheses. Vol. 77, pp. 481–487.
- ↑ 48.0 48.1 Pacurar R, Miclaus C, Miclaus M. Morbidity associated with breast cancer therapy and the place of physiotherapy in its management. Timisoara Physical Education & Rehabilitation Journal 2011 05;3(6):46-54.
- ↑ JOHNSON, S. and MUSA, I., 2004.Preparation of the breast cancer patient for radiotherapy planning. Physiotherapy. . Vol. 90, no. 4, pp. 195-203.
- ↑ Dahl AA, Nesvold I, Reinertsen KV, Fosså SD. Original Article: Arm/shoulder problems and insomnia symptoms in breast cancer survivors: Cross-sectional, controlled and longitudinal observations. Sleep Med 2011;12:584-590.
- ↑ Freitas-Silva R, de Freitas-Júnior, R. ( 1 ), Conde DM(2), Martinez EZ(3). Comparison of quality of life, satisfaction with surgery and shoulder-arm morbidity in breast cancer survivors submitted to breast-conserving therapy or mastectomy followed by immediate breast reconstruction. Clinics 2010 / 06 / 01 /;65(8):781-787.
- ↑ Harrington S(1), Padua D(2), Myers J(2), Battaglini C(3), Groff D(3), Michener LA(4), et al. Comparison of shoulder flexibility, strength, and function between breast cancer survivors and healthy participants. Journal of Cancer Survivorship 2011 / 06 / 01 /;5(2):167-174.
- ↑ Todd J, Topping A. A survey of written information on the use of post-operative exercises after breast cancer surgery. Physiotherapy 2005;91:87-93.
- ↑ ) Amaral MTPd, de Oliveira M,Maia Freire, Ferreira NdO, Guimarães R, Vidigal, Sarian L, Otávio, Gurgel MSC. Manual therapy associated with upper limb exercises vs. exercises alone for shoulder rehabilitation in postoperative breast cancer. PHYSIOTHER THEORY PRACT 2012 05;28(4):299-306.
- ↑ RafflesHospital. Breast self-examination (it can save your life). Available from: https://www.youtube.com/watch?v=LrfE6JUwIms&feature=emb_logo [last accessed 15/11/2018]
- ↑ Smith RA, Saslow D, Sawyer KA, Burke W, Costanza ME, Evans III WP, Foster Jr RS, Hendrick E, Eyre HJ, Sener S. American Cancer Society guidelines for breast cancer screening: update 2003. CA: a cancer journal for clinicians. 2003 May;53(3):141-69.
- ↑ 57.0 57.1 57.2 Allen TL, Van Groningen BJ, Barksdale DJ, McCarthy R. The breast self-examination controversy: what providers and patients should know. The Journal for Nurse Practitioners. 2010 Jun 1;6(6):444-51.
- ↑ 58.0 58.1 58.2 58.3 58.4 58.5 58.6 What You Need to Know About Breast Cancer. National Cancer Institute. http://www.cancer.gov/cancertopics/wyntk/breast/allpages. (accessed 21 February 2010)
- ↑ 59.0 59.1 59.2 59.3 59.4 59.5 59.6 59.7 Goodman CC, Fuller KS. Pathology: Implications for the Physical Therapist, 3rd Ed. St. Louis, MO: Saunders Elsevier, 2009, pp 1015-1036. (Loe 1b)
- ↑ Garduño-Ramón MA, Vega-Mancilla SG, Morales-Henández LA, Osornio-Rios RA. Supportive noninvasive tool for the diagnosis of breast cancer using a thermographic camera as sensor. Sensors. 2017 Mar;17(3):497.
- ↑ 61.0 61.1 61.2 BreastCancer.org. http://www.breastcancer.org/ (accessed 21 February 2010).
- ↑ 62.0 62.1 62.2 62.3 Panus PC, Katzung B, Jobst EE, Tinseley SL, Masters SB, Trevor AJ. Pharmacology for the Physical Therapist. Cancer Chemotherapy. New York: McGraw-Hill Companies, Inc., 2009. p460-477.
- ↑ Brouckaert O, Van Asten K, Laenen A, Soubry A, Smeets A, Nevelstreen I, Vergote I, Wildiers H, Paridaens R, Van Limbergen E, Weltens C. Body mass index, age at breast cancer diagnosis, and breast cancer subtype: a cross-sectional study. Breast cancer research and treatment. 2018 Feb 1;168(1):189-96.
- ↑ Ozmen V, Ilgun S, Ozden BC, Ozturk A, Aktepe F, Agacayak F, Elbuken F, Alco G, Ordu C, Iyigun ZE, Emre H. Comparison of breast cancer patients who underwent partial mastectomy (PM) with mini latissimus dorsi flap (MLDF) and subcutaneous mastectomy with implant (M+ I) regarding quality of life (QOL), cosmetic outcome and survival rates. World Journal of Surgical Oncology. 2020 Dec;18(1):1-2.
- ↑ FISHER, B., ANDERSON, S., BRYANT, J., MARGOLESE, R.G., DEUTSCH, M., FISHER, E.R., JEONG, J. and WOLMARK, N., 2002. Twenty-year follow-up of a randomized trial comparing total mastectomy, lumpectomy, and lumpectomy plus irradiation for the treatment of invasive breast cancer. New England Journal of Medicine. . Vol. 347, no. 16, pp. 1233-1241.
- ↑ 66.0 66.1 Beurskens CHG(1), van Uden, C.J.T. ( 1,2 ), Strobbe LJA(3), Oostendorp, R.A.B. ( 4,5 ), Wobbes T(6). The efficacy of physiotherapy upon shoulder function following axillary dissection in breast cancer, a randomized controlled study. BMC Cancer 2007 / 08 / 30 /;7.
- ↑ Yarnold J. Early and locally advanced breast cancer: diagnosis and treatment National Institute for Health and Clinical Excellence guideline 2009. Clin Oncol (R Coll Radiol) 2009 04;21(3):159-160.
- ↑ HICKEY, B.E., FRANCIS, D.P. and LEHMAN, M., 2013. Sequencing of chemotherapy and radiotherapy for early breast cancer. Cochrane Database Syst Rev. , vol. 4.
- ↑ Monsuez J, Charniot J, Vignat N, Artigou J. Review: Cardiac side-effects of cancer chemotherapy. Int J Cardiol 2010;144:3-15.
- ↑ Senkus E, Jassem J. Complications of Treatment: Cardiovascular effects of systemic cancer treatment. Cancer Treat Rev 2011;37:300-311.
- ↑ Yeh E. Cardiotoxicity induced by chemotherapy and antibody therapy. Annu Rev Med 2006;57:485-498.
- ↑ Johansen S, Foss K, Nesvold I, L., Malinen E, Foss S, D. Arm and shoulder morbidity following surgery and radiotherapy for breast cancer. Acta Oncol 2014 04;53(4):521-529.
- ↑ Schnur JB(1), Montgomery GH(1), Ouellette SC(2), Dilorenzo TA(3), Green S(4). A qualitative analysis of acute skin toxicity among breast cancer radiotherapy patients. Psychooncology 2011 / 03 / 01 /;20(3):260-268.
- ↑ Cox JD, Stetz J, Pajak TF. Toxicity criteria of the Radiation Therapy Oncology Group (RTOG) and the European Organization for Research and Treatment of Cancer (EORTC). Int J Radiat Oncol Biol Phys 1995 03/30;31(5):1341-1346.
- ↑ Pacurar R, Miclaus C, Miclaus M. Morbidity associated with breast cancer therapy and the place of physiotherapy in its management. Timisoara Physical Education & Rehabilitation Journal 2011 05;3(6):46-54.
- ↑ Pacurar R, Miclaus C, Miclaus M. Morbidity associated with breast cancer therapy and the place of physiotherapy in its management. Timisoara Physical Education & Rehabilitation Journal 2011 05;3(6):46-54.
- ↑ Nucleusanimation. Breast Cancer Surgery: Lumpectomy, Mastecomy. http://www.youtube.com/watch?v=WUoqsBxgXWI (accessed 7 April 2010).
- ↑ Patient Education: Breast Biopsy Surgery. http://www.youtube.com/watch?v=to6fPH4rQNQ (accessed March 22, 2012).
- ↑ YouTube. What is a skin-sparing mastectomy? http://youtu.be/Zs9E3Nd49Z0 (accessed 15 Mar 2012).
- ↑ Pacurar R, Miclaus C, Miclaus M. Morbidity associated with breast cancer therapy and the place of physiotherapy in its management. Timisoara Physical Education & Rehabilitation Journal 2011 05;3(6):46-54.
- ↑ Pariser D. Chemotherapy Power Point Presentation. PT 535 Pharmacology, Bellarmine University. Presented November 5, 2010.
- ↑ 82.0 82.1 Ranpura V, Hapani S, Wu S. Treatment-related mortality with bevacizumab in cancer patients: a meta-analysis. Jama. 2011 Feb 2;305(5):487-94.
- ↑ Cortes J, O'Shaughnessy J, Loesch D, Blum JL, Vahdat LT, Petrakova K, Chollet P, Manikas A, Diéras V, Delozier T, Vladimirov V. Eribulin monotherapy versus treatment of physician's choice in patients with metastatic breast cancer (EMBRACE): a phase 3 open-label randomised study. The Lancet. 2011 Mar 12;377(9769):914-23.
- ↑ McAnaw MB, Harris KW. The Role of Physical Therapy in the Rehabilitation of Patients with Mastectomy and Breast Reconstruction. Breast Disease 2002 12;16(1):163-174.
- ↑ Levangie PK, Drouin J. Magnitude of late effects of breast cancer treatments on shoulder function: a systematic review. Breast Cancer Res Treat 2009;116(1):1-15.
- ↑ EBAUGH, D., SPINELLI, B. AND SCHMITZ, K.H., 2011. “Shoulder impairments and their association with symptomatic rotator cuff disease in breast cancer survivors”, Medical Hypotheses. Vol. 77, pp. 481–487.
- ↑ Pacurar R, Miclaus C, Miclaus M. Morbidity associated with breast cancer therapy and the place of physiotherapy in its management. Timisoara Physical Education & Rehabilitation Journal 2011 05;3(6):46-54.
- ↑ Torres Lacomba M, Yuste Sánchez MJ, Zapico Goñi A, Prieto Merino D, Mayoral dM, Cerezo Téllez E, et al. Effectiveness of early physiotherapy to prevent lymphoedema after surgery for breast cancer: randomised, single blinded, clinical trial. BMJ (Clinical research ed ) 2010 / 01 / 01 /;340:b5396.
- ↑ 89.0 89.1 89.2 Courneya KS. Exercise in cancer survivors: an overview of research. Medicine & Science in Sports & Exercise 2003 11;35(11):1846-1852.
- ↑ Courneya KS, Friedenreich CM. Physical Activity and Cancer [electronic resource]. : Berlin, Heidelberg : Springer Berlin Heidelberg : Imprint: Springer, 2011; 2011.
- ↑ 91.0 91.1 91.2 PANEL E. American College of Sports Medicine roundtable on exercise guidelines for cancer survivors. 2010.
- ↑ Miedema B, Easley J. Barriers to rehabilitative care for young breast cancer survivors: a qualitative understanding. Supportive Care in Cancer 2012;20(6):1193-1201.
- ↑ Leach H, Danyluk J, Culos–Reed S. Design and implementation of a community-based exercise program for breast cancer patients. Current Oncology 2014;21(5):267.
- ↑ 94.0 94.1 94.2 94.3 94.4 94.5 Kärki A, Simonen R, Mälkiä E, Selfe J. Impairments, activity limitations and participation restrictions 6 and 12 months after breast cancer operation. Journal of rehabilitation medicine: official journal of the UEMS European Board of Physical and Rehabilitation Medicine. 2005;37(3):180-8.
- ↑ Cantarero-Villanueva I, Fernández-Lao C, Cuesta-Vargas AI, Del Moral-Avila R, Fernández-de-las-Peñas C, Arroyo-Morales M. The effectiveness of a deep water aquatic exercise program in cancer-related fatigue in breast cancer survivors: a randomized controlled trial. Archives of physical medicine and rehabilitation. 2013 Feb 1;94(2):221-30.
- ↑ Carter SJ, Hunter GR, McAuley E, Courneya KS, Anton PM, Rogers LQ. Lower rate-pressure product during submaximal walking: a link to fatigue improvement following a physical activity intervention among breast cancer survivors. Journal of Cancer Survivorship. 2016 Oct 1;10(5):927-34.
- ↑ Vardar Yağlı N, Şener G, Arıkan H, Sağlam M, İnal İnce D, Savcı S, Çalık Kutukcu E, Altundağ K, Kaya EB, Kutluk T, Özışık Y. Do yoga and aerobic exercise training have impact on functional capacity, fatigue, peripheral muscle strength, and quality of life in breast cancer survivors?. Integrative cancer therapies. 2015 Mar;14(2):125-32.
- ↑ Burnham TR, Wilcox A. Effects of exercise on physiological and psychological variables in cancer survivors. Medicine and science in sports and exercise. 2002 Dec 1;34(12):1863-7.
- ↑ McNeely ML, Campbell KL, Rowe BH, Klassen TP, Mackey JR, Courneya KS. Effects of exercise on breast cancer patients and survivors: a systematic review and meta-analysis. Cmaj. 2006 Jul 4;175(1):34-41.
- ↑ Campbell KL, Kam JW, Neil‐Sztramko SE, Liu Ambrose T, Handy TC, Lim HJ, Hayden S, Hsu L, Kirkham AA, Gotay CC, McKenzie DC. Effect of aerobic exercise on cancer‐associated cognitive impairment: A proof‐of‐concept RCT. Psycho‐oncology. 2018 Jan;27(1):53-60.
- ↑ Zhang Y, Xu L, Zhang X, Yao Y, Sun Y, Qi L. Effects of different durations of aerobic exercise on the cardiovascular health in untrained women: a meta-analysis and meta-regression.
- ↑ Roveda E, Vitale JA, Bruno E, Montaruli A, Pasanisi P, Villarini A, Gargano G, Galasso L, Berrino F, Caumo A, Carandente F. Protective effect of aerobic physical activity on sleep behavior in breast cancer survivors. Integrative cancer therapies. 2017 Mar;16(1):21-31.
- ↑ Yang HL, Hsieh PL, Hung CH, Cheng HC, Chou WC, Chu PM, Chang YC, Tsai KL. Early Moderate Intensity Aerobic Exercise Intervention Prevents Doxorubicin-Caused Cardiac Dysfunction Through Inhibition of Cardiac Fibrosis and Inflammation. Cancers. 2020 May;12(5):1102.
- ↑ 104.0 104.1 104.2 104.3 104.4 Cantarero-Villanueva I, Fernández-Lao C, del Moral-Avila R, Fernández-de-las-Peñas C, Feriche-Fernández-Castanys MB, Arroyo-Morales M. Effectiveness of core stability exercises and recovery myofascial release massage on fatigue in breast cancer survivors: a randomized controlled clinical trial. Evidence-Based Complementary and Alternative Medicine. 2012 Jan 1;2012.
- ↑ Clarkson PM, Kaufman SA. Should resistance exercise be recommended during breast cancer treatment?. Medical hypotheses. 2010 Aug 1;75(2):192-5.
- ↑ Shamley D, Lascurain‐Aguirrebeña I, Oskrochi R. Clinical anatomy of the shoulder after treatment for breast cancer. Clinical Anatomy. 2014 Apr;27(3):467-77.
- ↑ Giacalone A, Alessandria P, Ruberti E. The physiotherapy intervention for shoulder pain in patients treated for breast cancer: Systematic review. Cureus. 2019 Dec;11(12).
- ↑ McNeely ML, Campbell K, Ospina M, Rowe BH, Dabbs K, Klassen TP, Mackey J, Courneya K. Exercise interventions for upper‐limb dysfunction due to breast cancer treatment. Cochrane Database of Systematic Reviews. 2010(6).
- ↑ Galantino ML, Stout NL. Exercise interventions for upper limb dysfunction due to breast cancer treatment. Physical therapy. 2013 Oct 1;93(10):1291-7.
- ↑ Bertoli J, de Souza Bezerra E, Reis AD, da Costa Barros ÊA, Gobbo LA, Júnior IF. Long-Term Side Effects of Breast Cancer on Force Production Parameters. The Journal of Strength & Conditioning Research. 2020 May 13.
- ↑ Ottenbacher AJ(1), Day RS(1), Taylor WC(1), Sharma SV(1), Sloane R(2), Snyder DC(3), et al. Exercise among breast and prostate cancer survivors-what are their barriers? Journal of Cancer Survivorship 2011 / 12 / 01 /;5(4):413-419
- ↑ Blanchard CM, Courneya KS, Stein K. Cancer survivors' adherence to lifestyle behavior recommendations and associations with health-related quality of life: results from the American Cancer Society's SCS-II. J Clin Oncol 2008 2008;26(13):2198-2204.
- ↑ Turner, J. ( 1,4 ), Hayes S(2), Reul-Hirche H. Improving the physical status and quality of life of women treated for breast cancer: A pilot study of a structured exercise intervention. J Surg Oncol 2004 / 06 / 01 /;86(3):141-146.
- ↑ Monninkhof EM, Elias SG, Vlems FA, van dT, Schuit AJ, Voskuil DW, et al. Physical activity and breast cancer: a systematic review. Epidemiology 2007 01;18(1):137-157.
- ↑ Brunet J, Taran S, Burke S, Sabiston CM. A qualitative exploration of barriers and motivators to physical activity participation in women treated for breast cancer. Disability & Rehabilitation 2013 12/25;35(24):2038-2045.
- ↑ Blaney JM(1), Lowe-Strong A, Gracey JH(1), Rankin-Watt J, Campbell A(3). Cancer survivors' exercise barriers, facilitators and preferences in the context of fatigue, quality of life and physical activity participation: A questionnaire-survey. Psychooncology 2013 / 01 / 01 /;22(1):186-194.
- ↑ 117.0 117.1 HEFFERON, K., MURPHY, H., MCLEOD, J., MUTRIE, N., CAMPBELL, A., 2013. Understanding barriers to exercise implementation 5-year post-breast cancer diagnosis: a large-scale qualitative study. Health Education Research [online]. Vol 28, no. 5, pp. 843-6 [viewed November 2014]. Available from: NCBI.
- ↑ LOH, S. Y., CHEW, S., LEE, S., 2010. Physical Activity and Women with Breast Cancer: Insights from Expert Patients [online]. Vol. 11, pp. 87-4 [viewed November 2014]. Available from: NCBI.
- ↑ PARAMANANDAM, V. S., & ROBERTS, D., 2014. Weight training is not harmful for women with breast cancer-related lymphoedema: a systematic review. Journal of Physiotherapy [online]. Vol. 60, no. 3, pp. 136-3 [viewed November 2014]. Available from: NCBI.
- ↑ Emery, C.F. ( 1,2,3 ), Yang H-(1), Peterson LJ(1), Suh S(1), Frierson GM(4). Determinants of physical activity among women treated for breast cancer in a 5-year longitudinal follow-up investigation. Psychooncology 2009 / 01 / 01 /;18(4):377-386.
- ↑ Bower JE, Ganz PA, Desmond KA, Rowland JH, Meyerowitz BE, Belin TR. Fatigue in breast cancer survivors: Occurrence, correlates, and impact on quality of life. Journal of Clinical Oncology 2000 / 02 / 01 /;18(4):743-753.
- ↑ Hayes, S. ( 1,2,4 ), Newman B(1), Cornish B(3). Comparison of methods to diagnose lymphoedema among breast cancer survivors: 6-month follow-up. Breast Cancer Res Treat 2005 / 02 / 01 /;89(3):221-226.
- ↑ Hayes SC, Janda M, Cornish B, Battistutta D, Newman B. Lymphedema after breast cancer: Incidence, risk factors, and effect on upper body function. Journal of Clinical Oncology 2008 / 01 / 01 /;26(21):3536-3542.
- ↑ 124.0 124.1 124.2 PARAMANANDAM, V. S., & ROBERTS, D., 2014. Weight training is not harmful for women with breast cancer-related lymphoedema: a systematic review. Journal of Physiotherapy [online]. Vol. 60, no. 3, pp. 136-3 [viewed November 2014]. Available from: NCBI.
- ↑ Cheifetz O, Haley L. Management of secondary lymphedema related to breast cancer. Can Fam Physician 2010 12;56(12):1277-1284.
- ↑ MCKENZIE, D.C., A breast in a Boat - a race against breast cancer. Canadian Medical Assoc J [online]. Vol. 159, no.4, pp. 376-78 [viewed November 2014]. Available from: NCBI.
- ↑ Kwan ML, Cohn JC, Armer JM, Stewart BR, Cormier JN. Exercise in patients with lymphedema: a systematic review of the contemporary literature. JOURNAL OF CANCER SURVIVORSHIP-RESEARCH AND PRACTICE 2011;5(4):320-336.
- ↑ Schmitz KH(1), Troxel AB(1), Lewis-Grant L, Bryan CJ(1), Williams-Smith C, Chittams J(1), et al. Weight lifting for women at risk for breast cancer-related lymphedema: A randomized trial. JAMA - Journal of the American Medical Association 2010 / 12 / 22 /;304(24):2699-2705.
- ↑ Schmitz KH, Ahmed RL, Troxel A, Cheville A, Smith R, Lewis-Grant L, et al. Weight lifting in women with breast-cancer-related lymphedema. N Engl J Med 2009 08/13;361(7):664-673.
- ↑ LOH, S. Y., CHEW, S., LEE, S., 2010. Physical Activity and Women with Breast Cancer: Insights from Expert Patients [online]. Vol. 11, pp. 87-4 [viewed November 2014]. Available from: NCBI.
- ↑ 131.0 131.1 Burnham TR, Wilcox A. Effects of exercise on physiological and psychological variables in cancer survivors. / Effets de l ' activite physique sur les variables physiologiques et psychologiques des personnes en phase de remission d ' un cancer. Medicine & Science in Sports & Exercise 2002 12;34(12):1863-1867.
- ↑ 132.0 132.1 Brunet J, Taran S, Burke S, Sabiston CM. A qualitative exploration of barriers and motivators to physical activity participation in women treated for breast cancer. Disability & Rehabilitation 2013 12/25;35(24):2038-2045.
- ↑ Blaney JM(1), Lowe-Strong A, Gracey JH(1), Rankin-Watt J, Campbell A(3). Cancer survivors' exercise barriers, facilitators and preferences in the context of fatigue, quality of life and physical activity participation: A questionnaire-survey. Psychooncology 2013 / 01 / 01 /;22(1):186-194.
- ↑ World Health Organisation. The International Classification of Functioning, Disability and Health. 2001; Available at: http://www.who.int/disabilities/world_report/2011/report.pdf. Accessed 15 November, 2014.
- ↑ Halm MA. Clinical evidence review. Relaxation: a self-care healing modality reduces harmful effects of anxiety. Am J Crit Care 2009 03;18(2):169-172.
- ↑ Robotin M, Olver I, Girgis A. When cancer crosses disciplines : a physician's handbook. : London : Imperial College Press, c2010; 2010
- ↑ Unruh, A. and Elvin, N. (2004). In the eye of the dragon: Women's experience of breast caner and the occupation of dragon boat racing. R EVUE CANADIENNE D ’ ERGOTHÉRAPIE, 71(3), pp.138-149.
- ↑ Waddell, G., Burton, A.K and Kendall, N.A.S. Vocational Rehabilitation: What Works, for Whom and When?
- ↑ HIATT RA, RIMER BK. Principles and Applications of Cancer Prevention and Control Interventions. Cancer Epidemiology and Prevention 2006.
- ↑ Keeley V, Crooks S, Locke J, Veigas D, Riches K, Hilliam R. A quality of life measure for limb lymphoedema (LYMQOL). J LYMPHOEDEMA 2010 04;5(1):26-37.
- ↑ Mendoza TR(1), Wang XS(1), Cleeland, C.S. ( 1,4 ), Morrissey M(1), Johnson BA(1), Wendt JK(1), et al. The rapid assessment of fatigue severity in cancer patients: Use of the brief fatigue inventory. Cancer 1999 / 01 / 01 /;85(5):1186-1196.
- ↑ Yellen, S.B. ( 1,3 ), Cella DF(1), Webster K(1), Blendowski C(1), Kaplan E(2). Measuring fatigue and other anemia-related symptoms with the Functional Assessment of Cancer Therapy (FACT) measurement system. J Pain Symptom Manage 1997 / 02 / 01 /;13(2):63-74.
- ↑ Hudak, P.L. ( 1,2 ), Amadio, P.C. ( 1,3 ), Bombardier, C. ( 2,4 ). Development of an upper extremity outcome measure: The DASH (disabilities of the arm, shoulder, and head). Am J Ind Med 1996 / 01 / 01 /;29(6):602-608
- ↑ Zigmond AS, Snaith RP. The Hospital Anxiety and Depression Scale. Acta Psychiatr Scand 1983 06;67(6):361.
Top Contributors - Adam El-Sayed, Kim Jackson, Lucinda hampton, Vidya Acharya, Admin, 127.0.0.1, Chee Wee Tan, Ilona Malkauskaite, Candace Goh and Manisha Shrestha
Introduction[edit | edit source]
There is a growing evidence base reporting the physiological and psychological benefits of physiotherapy as a safe and effective adjunct to breast cancer treatment [1]. With survival rates at an all time high the National Cancer Survivorship Initiative (NCSI) Vision (Department of Health) has stated that health professionals must now focus on meeting the unique needs of breast cancer survivors and improve accessibility to specialist services, including physiotherapy. Services ideally will be able to deliver physiotherapy interventions to better empower patients in the management of their symptoms, side-effects of treatment or recovery from surgery. Recent specialist breast care physiotherapy services are now being developed so that physiotherapists are prepared to deliver a high standard of care through such initiatives.
Breast cancer patients face an array of problems and have specific needs which must be addressed in order to prevent long term functional limitations and disability. The physiological and psychological benefits from physiotherapy for breast cancer patients are well documented, with improvements observed in terms of morbidity, mortality and importantly, quality of life [2]. Thus, the potential role for physiotherapists in this area is clear to see and highlights the importance of training for physiotherapists to develop the skills required to meet patient needs and maximise their contribution to the MDT.
Breast Cancer Prevalence and Survival Rates[edit | edit source]
Breast cancer is the most common cancer in the UK. An estimated 1 in 8 women in the UK will develop breast cancer in their lifetime and the incidence of breast cancer has risen by 7% in the last 10 years. In part this may reflect the effectiveness of NHS screening programs (16,500 cases were detected in 2009 alone) however increasing incidence is also a global issue with an estimated 1.68 million diagnoses made in 2012 worldwide [3]. Survival rates are also increasing. Today, more women are surviving breast cancer than ever before. Most recent figures reported by Information Services Division Scotland showed that high percentages of women diagnosed with breast cancer in Scotland between 2003 and 2007 were surviving 1 year (93.8%) and 5 years post diagnosis (81.4%). Similar trends have been reported for the rest of the UK [3].
Pathophysiology of Cancer Cells[edit | edit source]
Cancer cells differ from normal cells in the disregulation of cell division and growth. As a normal cell develops into a neoplastic state, the cell undergoes changes in six forms.
- Sustaining proliferation: cancer cells change growth promoting signals by altering pro-ocogenes to oncogenes thereby disrupting the homeostasis of cell structure and function. This results in sustained chronic proliferation without external stimulation.
- Evading growth suppressors: by overcoming the effects of tumour suppressing genes that prevent cell growth, tumours are able to grow uninhibited.
- Resisting cell death (apoptosis): the spread of cancer cells may be escalated due to a rise in gene mutations leading to ineffective programmed cell death.
- Enabling replicative immortality: in normal cells telomere shortening causes cell division to stop. Cancer cells enable widespread self-replication using the enzyme telomerase to prevent telomere shortening, allowing repeated cell division without DNA shortening.
- Sustained angiogenesis: as in normal cells angiogenesis allows cancer cells to acquire nutrients and oxygen with the elimination of metabolic waste and carbon dioxide. Subsequently a vascular system is created to maintain tumour growth and metastasis.
- Activating invasion and metastasis: the spread of cancer cells is made possible when cells break free from the primary tumour and enter the blood and lymphatic vessels. Subsequently the development of a second tumour may occur in different site of the body to the primary tumour [4].
Pathophysiology in Breast Cancer[edit | edit source]
- Non-invasive breast cancers are present in the ducts or lobules.
- Invasive cancers are found in the surrounding breast tissue.
- Cell Grade: Cells are graded in a system where grade 1 cancer cells present slightly differently to normal cells, progressing to grade 3 cancer cells which demonstrate major differences to a normal cells.
- Tumour Necrosis: tumour necrosis may be present in aggressive forms of breast cancer where cells are seen to grow at a rapid rate.
- This is often a sign of a rapidly growing aggressive form of breast cancer.
- Vascular or Lymphatic Invasion: - these types of invasion describe whether or not cancerous cells are evident in the vascular and lymphatic vessels supplying the breast tissue.
- Hormone Receptor Status: - Hormone receptor status determines if hormone therapy would be appropriate.
- HER2 Status: - HER2 is a gene that when dysfunctional can play a role in the development of breast cancer. Breast cancers that are HER2 positive tend to grow faster and are more likely to spread that those that are HER2 negative.
Musculoskeletal Problems Experienced in Breast Cancer Patients[edit | edit source]
The breast cancer patient can also be susceptible to the development of musculoskeletal problems, the same as the general population. One common problem is symptomatic rotator cuff disease, which can be brought on through intrinsic factors such as age related physiological changes to the tendons, or through extrinsic factors brought on from cancer treatment such as lymphedema as well as shoulder girdle resting alignment. Tension overload on the rotator cuff tendons may be increased secondary to increased volume and weight of the effected limb with the presence of lymphedema. Due to pain, or fear of movement, for example, the breast cancer patient may adapt to a new resting position for their shoulder, and may tend to avoid using the limb, resulting in shortening of the muscles, and tightening of the joint capsule [5]. Moreover, patients tend to adapt a flexed and protective posture following surgery, further increasing the likelihood of muscle shortening. Pectoralis major is commonly effected. Tightness of these muscles tend to lead to a pull on the scapula, causing it to become protracted and depressed, leading to scapular winging, as well as shoulder impingement [6].
Post Mastectomy Pain Syndrome (PMPS)[edit | edit source]
Pain which lasts longer than what is usually expected following various breast cancer surgery types. Generally neuropathic in nature, and can be due but not limited to:
- Brachial nerve damage, *Intra-operative compromise of cutaneous innervating,
- Neuroma formation,
- Fibrotic entrapment. Patients often report neurological symptoms such as numbness or pins and needles, stabbing and burning pain to the same side as surgery in or around the surgical sites.
These symptoms can be exacerbated through a lack of pacing, or by lying on the side of surgery. Therefore, patient education, soft tissue massage, and other desensitising techniques are essential [6]. Other common problems include:
- Subacromial Impingement Syndrome.
- Adhesive Capsulitis (frozen shoulder) – idiopathic or traumatic (post-surgery).
- Rotator Cuff pathology (e.g Symptomatic Rotator Cuff Disease)
- Myofascial Dysfunction Lateral epicondylitis.
- Scapular winging secondary to damage of long thoracic nerve during surgery.
- Pain
Associated Neuromusculoskeletal Conditions Post Treatment[edit | edit source]
Neuromusculoskeletal conditions are common following surgery, some of which are illustrated in figure 1.4. Treatment protocols shall not be discussed, and the reader should refer to the basic principles of rehabilitation of musculoskeletal conditions. In light of this, it is important to briefly discuss a few points to consider.
- Depending on the type of surgery that the patient needs to undertake, radiotherapy may be necessary following surgery.
- A typical radiotherapy session will require the patient to position the treated arm to 90° flexion and abduction, as well as maximal external rotation, for up to 30 minutes [7].
- Shoulder mobility is commonly affected post-surgery [8]; [9]; [10] so it is vital that physiotherapy aims to restore this to improve patient functional ability and to be able to place the shoulder in the required positions for radiotherapy.
- Active, active assisted, and passive ROM exercises for the shoulder girdle are therefore good practice. Physiotherapy should aim to restore full shoulder ROM as well as minimising associated upper extremity morbidity [11].
- Manual therapy techniques with the aim of further increasing available ROM have been shown to not be of any significant benefit when used in conjunction with active upper limb exercises [12].
Medical Management[edit | edit source]
Due to the complex nature of cancer and the treatment process that accompanies the condition, the condition requires expert input across of wide range of health professionals [13]. In light of this, there is potential for miscommunication on occasion. A solid MDT should improve communication between health professionals, which should optimize the treatment process for the breast cancer patient [14].
Surgery[edit | edit source]
Surgery is performed in order to remove as many cancer cells as possible, to investigate where the cancer cells have spread to, for reconstruction, or to reduce symptoms in the later stages of cancer. Below is a brief outline of the common procedures. Selection criteria for surgery:
- The size of the tumour present and the location
- Whether or not the cancer cells have spread
- Breast size
- Personal wishes of the patient
Excisional Breast Biopsy (Lumpectomy)[edit | edit source]
- A lumpectomy, otherwise known as a wide local excision, tends to be used in early stage cancer or when the tumour is of small size.
- The surgeon will remove the cancer cells, as well as some of the healthy tissue which surrounds the affected area. *This can be done with general or local anaesthetic, with the aim of determining the mass of the tumour and to rule out any carcinoma.
- Due to the conserving nature of this procedure, it is one that is favoured by the breast cancer population, and has even been shown to have no significant difference in survival rate when compared with a mastectomy [15].
- The excised tumour is examined further for cancerous cells, if which are found around the edges, may lead to further surgery or mastectomy
Subcutaneous Mastectomy (Complete Mastectomy)[edit | edit source]
- This procedure results in complete removal of the breast tissue only (>99% of breast tissue), where a carcinoma is in situ.
- When the size of the tumour is large in proportion to the rest of the breast tissue, or is wide spread, a mastectomy is performed.
- As previously mentioned, a mastectomy may be performed if a lumpectomy is unsuccessful in the removal of all cancer cells.
- It may also be performed if cancer cells were to recur following other treatments such as radiotherapy.
*Occasionally, complete removal of the pectoralis muscle group can occur if cancer cells were to spread into the musculature.
Modified Radical Mastectomy (MRM)[edit | edit source]
- MRM indicated where:
- Tumour location, size, and presence of multiple cancer cells in the breast tissue.
- Where radiotherapy is contraindicated.
- Dissection and removal of the lymph nodes within the axilla occurs. The likeliness of morbidity in the arm [16] is increased and can even lead to permanent lymphedema depending on the amount of lymph nodes removed.
- Dissection sites:
- Lateral edge of the sternum, clavicle, within latissimus dorsi, rectus sheath
- Breast tissue’s usually dissected from the pectoralis muscle; occasionally compromised.
- Strain injuries to the brachial plexus are common, but usually resolve over time.
- Injuries to the long thoracic nerve are quite common following this particular surgery.
- Loss of range of motion (ROM) in the glenohumeral joint (GHJ) is quite common following this procedure, physiotherapy has been shown to manage this [16].
Sentinel Lymph Node Biopsy[edit | edit source]
- Radioactive dye is injected into the breast tissue as a further diagnostic measure to evaluate whether the cancer cells have entered the lymph nodes or not.
- Once identified, the lymph nodes containing the cancer cells, i.e. the sentinel lymph nodes, are then removed surgically.
Radiotherapy[edit | edit source]
- Almost half of cancer patients will use radiotherapy over the course of their cancer treatment.
- Designed to eliminate cancer cells through the application of high-radiation energy to the affected site. *One the main causes of arm pain in cancer patients [17]
- Risk factor for breast cancer related lymphedema.
- It is often used in with and separately to chemotherapy
- Radiotherapy can be used as a neoadjuvant treatment, or as an adjuvant treatment.
- The main muscles in the line of radiotherapy are the pectoralis muscles, latissimus dorsi, and serratus anterior. Although radiotherapy is a successful tool in the treatment of cancer, there are quite a few side effects, which may last days to years. Skin irritation, for example, is one of the most common side effects [18], with patients experiencing erythema, skin peeling, and even necrosis [19]. As previously mentioned, the direct contact radiotherapy has on the muscles pectoralis major, latissimus dorsi, and serratus anterior, can lead to damage of these healthy tissues, causing pain and inflammation. As in relation to the post-surgical posture which was previously mentioned, patients can tend to adapt a protective posture due to increased sensitivity and fear of movement, again predisposing to contractures and a decreased ROM [6].
Radiotherapy Side Effects[edit | edit source]
Radiation Induced Fibrosis (RIF)[edit | edit source]
RIF is common following high dose radiotherapy which causes secondary damage to the normal tissues which surround the cancer sites. 45% of patients report RIF related breast pain, which in turn negatively effects the patients’ quality of life [6].
Chemotherapy[edit | edit source]
- Chemotherapy is a systemic therapy with the aim of killing the dividing cancer cells present in the tissue through the use of a single of various different drugs.
- Adjuvant chemotherapy prolongs disease free and overall survival in patients with early breast cancer [20], and is primarily used in the earlier stages of the disease.
- Used if cancer cells have spread from the original site, or if there is risk that it will spread.
- It can be used as a curative therapy, as well as in conjunction with radiotherapy [21], or can be used to help relieve some symptoms present in the later stages of cancer.
- Treatment processes usually last up to 6 months.
- Chemotherapy may involve the use of a single drug, or a combination of different drugs. A full list of drugs used in cancer treatment, along with their side effects, can be located here.
The benefit of chemotherapy is evident, in contrast, cardiac side effects are also quite common amongst patients treated with chemotherapy. Depending on the drug used, various cardiac side effects can develop, such as:
- Myocardial ischemia
- Left ventricle dysfunction Although these issues cannot be directly influenced through physiotherapy, knowledge of the potential presence of these conditions is vital [22]. Chemotherapy induced cardiac toxicity is an ever growing problem, with research stating patients receiving chemotherapy are placed at Stage A heart failure, increasing the risk of cardiac dysfunction in the future [23], with the chance of heart failure possibly being higher than the chance of cancer recurrence [24]
Chemotherapy Side Effects[edit | edit source]
Hormone Therapy[edit | edit source]
Hormone therapy will only work in oestrogen receptor positive cancers. Oestrogen receptor positive cancer cells contain a hormone receptor which enables the binding of oestrogen onto the cancer cells, enabling further growth of the cancer. These same receptors are present on normal and healthy breast tissue cells. Hormone therapy aims to:
- To limit growth of the spreading hormone positive cancer cells
- To prevent recurrence
- To decrease the risk of developing hormone positive cancers in the future Aromatase inhibitors are drugs used in hormone therapy. Aromatase is involved in the biosynthesis of oestrogen from androgens located in various tissues throughout the body, primarily adipose tissue in menopausal women. Aromatase inhibitors also lead to a loss of bone density, further predisposing the breast cancer patient to osteoporosis. Therefore, the role of the physiotherapist in hormone therapy should be advice and education, accompanied with cardiovascular and light resistance training with the aim of decreasing chance of obesity and lowering the stress on the potentially weakened bones [6].
Hormone Therapy Side Effects[edit | edit source]
Like with chemotherapy, there are many different medications used in hormone therapy, each of which have different side effects.
Complimentary Therapies[edit | edit source]
There are many therapies which could possibly be discussed with a breast cancer patient, with the aim of helping them cope through their rehabilitation, any negative symptoms, and to promote their general well-being.
Musculoskeletal Physiotherapy Interventions Post Surgery[edit | edit source]
A physiotherapists treatment plan should include:
- Motion exercises to improve tissue extensibility and facilitate normal movement patterns.
- Myofascial release for enhancing mobility and enhancing tissue extensibility. [25] [26] [5] [6]
Reduced Range of Motion and Lymphedema[edit | edit source]
The two most common complications that are evident in breast cancer patients are restricted arm motion and lymphedema. These result in the occurrence of pain at the place of operation and muscle spasms are quite common. It is very important that early rehabilitation is implemented to promote functional movement to the patient’s previous level of activity. Subsequent to operation arm mobilisations are implemented first or second day post-op. Mobilisations are performed using joint rotations to tolerance but abduction and flexion are limited to 40°. At day 4 post-op flexion and abduction are gradually increased to 45°, this can be increased furthermore by 10-15° per day dependent on the patient’s pain tolerance. The technique performed by holding the patients arm in 45° flexion or abduction until the drains are removed. Secondary lymphedema is a common occurrence in the breast cancer population following surgery and has a long term negative effect on patient quality of life. Risk factors that are prevalent in the breast cancer population are age, obesity and the growing survival rate. The growing risk factors make secondary lymphedema a challenging complication in the breast cancer population.
Physiotherapy Interventions for Lymphedema[edit | edit source]
Complex decongestive physiotherapy for lymphedema[edit | edit source]
This is a treatment that incorporates skin hygiene, manual lymph drainage, bandaging, exercises and support garments. Manual lymphoedema drainage is a massage technique that involves the skin surface only. This follows the anatomical lymphatic pathway. Generally the manual lymph drainage technique will begin centrally in the neck and trunk to alleviate any lymphedema in the main lymphatic pathway, so that drainage in the arm is facilitated. Complex decongestive physiotherapy has been suggested as the primary treatment for breast cancer patients. This treatment includes skin care, exercises, compression and manual lymphedema treatment. Complex decongestive physiotherapy has shown to be an effective method for the treatment of lymphedema when standard elastic compression has been unsuccessful. One study showed consistent results of the reduction in volume of the effected extremity in 95% of 400 patients. A follow-up showed that these therapeutic results were maintained at 3 years.
Elevation[edit | edit source]
Elevation has been described as a non-effective treatment when performed solely in breast cancer treatment for arm related oedema. Elevation is commonly used together with other treatments to provide the most effective treatment. Most commonly in breast cancer treatment a multidisciplinary approach is taken where the patient will undergo massage and exercise. A specific technique of massage is commonly implemented named manual lymphatic drainage. Manual lymphatic drainage is a type of massage used to mobilise oedema fluid from distal to proximal areas and from areas of stasis to healthy lymphatics.
Compression[edit | edit source]
One form of lymphedema treatment which has proved most effective in the treatment of breast cancer patients is the use of standard elastic compression garments. Studies have shown significant improvements using simple elastic compression treatment for lymphedema, where 34% of patients showed a substantial reduction in arm oedema at 2 months and 39% of patients at 6 months. This conclusion was also seen in patients older than 65 years old [27].
Physiotherapy to prevent secondary lymphedema arising[edit | edit source]
- Stretching for muscles of the shoulder: Levator scapulae; upper trapezius; pectoralis major and the medial and lateral rotators of the shoulder
- Progressive active and active assisted shoulder exercises.
- Functional exercise activities.
- Proprioceptive neuromuscular facilitation exercises.
Physical Activity[edit | edit source]
Exercise is increasingly being implemented as a therapeutic tool in patients with breast cancer [28]. In recent times it has become clear that exercise has a central role to play in controlling and preventing chronic illness. However, statistically breast cancer survivors have a very low compliant rate and despite the renowned benefits of exercise, 22% Canadian cancer survivors report being inactive [29]. Physical exercise has shown to be a suitable adjunct therapy to battle long term chronic conditions and has been successful in reducing mortality and improving overall quality of life. There is substantial evidence to support the benefits of exercise in breast cancer in both during and after chemotherapy. Research has shown that physical activity and exercise is effective in improving QoL, cardiorespiratory fitness, physical functioning in breast cancer patients and survivors [30]. These studies where compared with similar studies and analysed in separate components such as safety, aerobic fitness, muscular strength, body size and composition, QOL, fatigue, anxiety. Physical exercise has shown to be a suitable adjunct therapy to battle long term chronic conditions and has been successful in reducing mortality and improving overall quality of life. A review of the current literature by Corneya [28] carried out an overview of research on the effect of exercise on cancer. Twelve studies met the inclusion criteria and showed statistically significantly beneficial results in the effects of exercise during breast cancer. More so, the studies demonstrated benefits exercise capacity, body weight and overall quality of life. The ACSM [30] discussed guidelines for cancer survivors and analysed studies during and after breast cancer separately. The ACSM[30] compared studies during breast cancer using the following headings; safety, aerobic fitness, muscular strength, body size and composition, QOL, fatigue, anxiety.
Precautions[edit | edit source]
There is a general assumption that upper limb exercises could be harmful to patients who had axillary nodes removed or radiation the axilla, however the latest research does not support this view.
When performing exercise for post surgical populations the SEWS chart should be monitored regularly for early warning signs. If the patient is feeling fatigued or anaemic exercise should be delayed.
Exercise Prescription[edit | edit source]
The ACSM guideline suggests that an overall volume of 150 mins of moderate-intensity exercise or 75 min of vigorous intensity exercise. Resistance exercise should be performed 2-3 times weekly with exercises including the major muscle groups. However, with patients undergoing cancer treatment the key is to avoid inactivity and the patient should remain as active at their ability and condition allows them.
FITT Guidelines[edit | edit source]
There is a huge gap at present in the research governing exercises prescription and its translation to the real world and community settings. As discussed earlier exercise compliance post cancer is very low and a qualitative study by Miedema [31] stated numerous factors for this such as lack of availability of services, travel issues, cost and personal reasons as family responsibilities and fatigue. Physiotherapist should be aware of the barriers to exercise compliance in this specific population (See #Barriers ).
The beauty program aims to counteract key concerns associated with breast cancer patients such as fatigue, reduced QoL, social anxiety and physical conditioning. Considering there is huge physiological benefits as well major psychological benefits it is important that the physiotherapist promotes the benefits of exercise immediately post-surgery and ensures that the exercise program is assessable at home or in the community and is specific to the individual. All exercise programs should be designed with F.I.T.T principles during and after breast cancer. A recent study by Leach [32] designed an exercise program called BEAUTY (The breast cancer patient engaging in activity and undergoing treatment program).The beauty program aims to bridge the gap between the body of evidence supporting the role of exercise in breast cancer and implementing this in a real world setting.
BEAUTY:
Patients were assessed by exercise physiologist before undergoing exercise treatment and underwent a variety of tests to get measure of baseline fitness and also filled out questionnaires relating to exercise and medical history, fatigue, QoL and depressive symptoms.
FITT Principle After Breast Cancer[edit | edit source]
- Warm up: 5-10 minutes to raise heart rate
- Aerobic Exercise: Frequency:
- 3 x 5 times per week **Intensity: 50-70% of max. heart rate
- Type: walking cycling aerobic activity
- Time: 30 minutes maintaining as a long term routine
- Resistance Training: Frequency:
- 2/3 times a week
- Intensity: 12/15 reps of 60 % of 1RM
- Type: Supervised resistance program of major muscle groups
- Time: 6 weeks
Barriers, Motivators and Myths and Physical Activity[edit | edit source]
- Breast cancer patients report more barriers to exercise than other cancer groups [33].
- The barriers are having a clear impact on activity participation.
- Only 30-47% of cancer survivors are undertaking the physical activity guidelines of 30 minutes of moderate intensity activity per day [34]
- Understanding the barriers can help physiotherapists in making services more accessible to breast cancer survivors and encourage participation.
Myths[edit | edit source]
Physiotherapists have an important role to play as educators in dispelling the myths and presenting patients with evidence to reassure them that exercise will not be harmful. Survivors often report concerns about the safety and benefits of exercise for their condition, however it is important to note that exercise both during and after cancer treatment has no known significantly negative effects [28] [35] [36].
Barriers[edit | edit source]
The main themes identified from interviews with survivors are physical, environmental and psychosocial [37]. Top 10 patient reported barriers to exercise [38]
- Concerns about their illness and other health problems
- Joint stiffness
- Fatigue
- Pain
- Lack of motivation
- Weather
- Lack of facilities
- Weakness
- Lack of interest
- Fear
Psychological Barriers[edit | edit source]
Psychological barriers identified from a large qualitative study by Hefferon et al [39] included:
- Lack of motivation
- Fear of injury
- Fear of lymphodema
- Dislike of gym environment
- lLck of privacy
“I just couldn’t … I had no energy to do anything. I could hardly drag one leg after the other...” - Anon [39]
"I had my breast off, I had to go through a year later for another operation … you’re all kind of sore and tense [...] plus the fact that the chemotherapy affected the nerve in my feet, so I didn’t have the strength ... I didn’t have the feeling in my feet.” - Anon (Hefferon et al. 2013)
Low mood and depression are commonly reported by patients and understandably make motivation to exercise extremely difficult.
“I feel sad. Even when I wanted to go out.…With other kind of physical sickness it’s ok but with cancer… so difficult to get myself out of this…” -Anon [40]
Physical Barriers[edit | edit source]
The following may contribute to negative feelings towards exercise participation [41]:
- Cancer treatment side-effects including weakness, loss of shoulder movement, musculoskeletal pain, neuropathy, weight gain and breast cancer related lymphoedema
- Post surgery complications e.g numbness, shoulder stiffness, lymphodema [42]
- Cancer or age related aches and pains
- More than half of patients report fatigue and 68% of participants in one study reported not having been told how to manage it [43]
Breast Cancer Related Lymphodema (BCRL)[edit | edit source]
BCRL manifests as chronic oedema of the upper limb and trunk in response to damaged axilliary lymph nodes as a result of surgery. As a result patients experience pain, a heavy feeling in the arm, psychological distress and self image concerns as well as increased risk of infection [44] [45]. Occurrence differs for populations of survivors, with prevalence being reported as anything between 2 and 83% [41]. In the past it was common belief that exercise could exacerbate BCRL and as such women were discouraged from exercises such as weight training [46], [41]. Research inspired by McKenzie et al's [47] “Abreast in a Boat – a race against breast cancer” has since refuted this, leading to a new culture of exercise promotion in breast cancer survivors.
Dragon Boat:
The subject is still a source of confusion and concern for many survivors. At present it cannot be said for certain that exercise will not increase occurrence of BCRL, however recent research does suggest that exercise does not cause clinically harmful effects related to BCRL [48],[41]. Encouragingly, studies on weight training and BCLR found no significant link between exercise and increased oedema when the exercises were closely supervised and progressed gradually [49] [50]. Furthermore, the implementation of early physiotherapy may lower or eliminate the occurrence of secondary lymphoedema in breast cancer patients who have undergone surgery.
Psychosocial Barriers[edit | edit source]
Patients may have Psychosocial barriers to exercise such as time constraints, issues with return to work, family commitments, as well as the facilities and environments available for exercise participation. Cancer incidence is high in women aged between 40-50. Many in this age group are active, working mothers with families and many responsibilities who devote much of their time to family and child care. We must also consider the time demands that come with multiple hospital appointments during and after treatment. As therapists we must therefore consider where and when the patient can exercise, what alternatives and comprises can be made as an alternative to gym based exercise.
Barriers During Versus After Treatment[edit | edit source]
Recently diagnosed women expressed more negative feelings towards physical activity than those after treatment, and may be less aware of the benefits of activity. Those who were currently undergoing treatment state that the need to conserve energy and fear of infection were of great concern to them when deciding to exercise. [51].
Motivators[edit | edit source]
Social support is commonly reported as a motivator for survivors. Group programs are said to provide a supportive, safe environment [52], which therapists should keep in mind when offering exercise advice.
“I think it is good to find a group of people and do together …because being alone is very lonely and harder to sustain the physical activity.’-Anon [52]
Common motivators reported include weight loss, health benefits, increased energy, body image and social support [37]. One patient stated the importance of exercise facilitators being understanding and caring.
“...the instructor that I had was very caring. I couldn’t keep up through the whole class so she would come up to me and say: ‘Are you doing ok?’ I think that helped me. It made me feel, not that people felt sorry for me, but were concerned that I was okay.” Anon [37]
Top 10 patient reported motivators for exercise [38]
- Fun
- Variety of exercises
- Gradual progression
- Flexible
- Personal goal setting
- Good music
- Individually tailored
- Feedback given
- Oncologist approval
- GP approval
Psychological Issues[edit | edit source]
Breast cancer diagnosis is a traumatic experience affecting not only the patient but all involved in the patient’s life. Cancer patients go through an emotional journey that affects their psychological wellbeing and mental health. It is therefore important that alongside the physiological treatment, care is taken of the patient’s emotional wellbeing to protect their long-term quality of life. The psychological issues surrounding the breast cancer patients, their carers and health workers, should be addressed at an early stage by ensuring that support and guidance is available to patients and others in need.
Biopsychosocial Model[edit | edit source]
The World Health Organization defines the biopsychosocial model as:
“attempts to achieve a synthesis, in order to provide a coherent view of different perspectives of health from a biological, individual and social perspective” [53]
The goal of the ICF classification is to provide a unified language and framework to describe health and health-related states. The ICF has two parts:
- Functioning and Disability - includes body function and structures, and activities and participation. #Contextual Factors - includes environmental factors, as well as personal factors. These represent the individual’s background and lifestyle. The environmental factors are the physical, social and attitudinal environment in which the person lives. They are external factors and can either be positive or negative influences on the individual’s performance as a member of society, on the individual’s ability to complete tasks, or on the individual’s body function and structure. (see #Barriers.2C_motivators_and_myths_behind_physical_activity_in_breast_cancer_survivors )
Environmental Factors[edit | edit source]
These include the immediate environment e.g home, workplace or school. Other individuals who whom they have direct contact are also included. The second focus is the way the environmental factors relate to functioning and disability. Different environments may produce different impacts on the same individual with a given health condition. An environment with barriers will restrict an individual’s performance in society, while an environment that is more facilitating may enhance their performance. (see #Barriers.2C_motivators_and_myths_behind_physical_activity_in_breast_cancer_survivors )
Personal Factors[edit | edit source]
This includes important aspects of the individual not directly related to their health status. They include things such as gender, race, age, other health conditions, fitness, lifestyle, habits, upbringing, coping styles, social background, education, profession, past and current experience, overall behavior pattern and character style, individual psychological assets and other characteristics, all or which may play a role in any level of disability. The ICF is based on the integration of the functioning and disability model with contextual factors. This is in an attempt to capture the integration of various perspectives of functioning. Thus, it attempts to achieve a synthesis, in order to provide a coherent view of different perspectives of health from a biological, individual and social perspective. As such the biopsychosocial model provides a framework to assist healthcare professionals in “expanding their repertoire of anxiety management strategies and empowering patients to learn self-healing modalities that interrupt major anxiety pathways, thereby contributing to secondary prevention.” [54] Working through the biopsychosocial model of health, it is clear that in our role as physiotherapists we need to address more than just patient’s physical needs. All the needs and concerns need to be treated.
The advancement of science and medical technology has brought a decrease in physical deformity from today’s surgical treatments. said, contemporary breast cancer treatments are becoming more complex and require a longer period of time. This is due to an increase in the available information and due to more opportunities of information and choices given to patients detailing their cancer diagnosis and prognosis and sharing the decision-making process regarding clinical intervention and treatment.
Psychological Aspects[edit | edit source]
Amongst the psychological aspects impacting on the physiological system are depression, self-esteem, fear of recurrence, changes in body image and sexuality, as well as physical toxicities that result from adjuvant therapy. A research carried out by Ganz, P.A. (2008) addresses the importance of identifying the psychological and social concerns of breast cancer patients in the clinical setting, and it assists patients in obtaining appropriate psychosocial services. Further information from this paper can be obtained by following this link. Robotin M. et al [55] discuss the impact that the psychological concerns have on cancer survivors and state that:
“other issues such as difficulties in decision-making and social isolation may emerge months or years after treatment has finished”
This implies the nature of the survivorship – a dynamic experience which changes over time – and the importance of the life style and social support to cancer survivors. Despite recent advances in the treatment of cancer, people have a universal dread of cancer and in most cases the disease remains highly stigmatised.
Strategies for Dealing With Psychological Issues[edit | edit source]
The following are some of the key strategies we should take into account when addressing the psychological problems that may arise from breast cancer diagnosis:
Patient-Centred verbal communication is key and all staff should seek to develop their skills in this aspect as part of the continuous professional development. All forms of communication should be patient-centred. Each health professional should act pro-actively to develop good working relationships with key relevant players and patients, including MDTs. To address and overcome these psychological issues it is imperative to have effective communication tools that can be used and accessed by all those involved in the patient health journey.
Physiotherapy - Long Term Management[edit | edit source]
When exploring and defining strategies for long-term physiotherapy management of patients with breast cancer, barriers, motivators and myths behind physical activity in breast cancer survivors should also be addressed.
The role of a physiotherapist is to promote a healthy life style including physical activity and proper nutrition. As highlighted previously, exercise interventions are being used and incorporated into treatment however much more research is needed to implement it on a higher standers of care level. Continuation of exercise can continue to foster motivation in patients, provide a support group for patients, enable social and psychological wellbeing. Most of all it can improve patients quality of life. It allows patients to have some control over their lives, stability and routine. It allows them to regain themselves and return to being active in a community [56].
Education[edit | edit source]
Education plays a vital role in patient centred care and clinician evidence base practice. There is clear efficacy for the use of education as has been highlighted throughout this resource. Breast Cancer Care developed a resource for exrcise after breast cancer surgery and is readily available here. McMillan Cancer Support also have another useful resource targeted towards marketing activitiy entitled Move More
The ACPOHE state that education of the patient is a key component of the physiotherapists role. ACPOHE recommend promotion of physical activity, independence and self-management as greatly important for successful rehabilitation outcomes. In reference to the biopsychosocial model of health, it is clear that physiotherapists have a duty to address more than just the patient’s physical problems. All patient needs and concerns need to be treated, an issue which was highlighted by Karen Middleton (CSP Chief Executive) in a speech at the Physiotherapy UK 2014 conference which may be viewed here.
“...physios can reverse injury, enable people to live with long-term conditions, integrate health and social care and help people back into work...we’re very good value”
Life After Cancer[edit | edit source]
Life after breast cancer treatment means returning to some familiar things and also making some new choices. The end of treatment does not mark the end of the journey with breast cancer. The patient embarks to adjusting to life as a breast cancer survivor and in many way will have a life that is in some ways very different from the life before. These changes include relationships to eating habits and exercise. How do you fight lingering fatigue? What should you eat to help prevent a breast cancer recurrence? Will you ever have a regular sex life again? These are just a few of the questions that may nag at the patient as they make the transition from breast cancer treatment to breast cancer survival. The patient’s body has been through an enormous assault and recovery is a huge thing – the patient cannot bounce back right away. Two of the more frustrating and troubling side effects women face after treatment are fatigue resulting from chemotherapy and/or the accumulated effects of other treatments, and a phenomenon some women have dubbed "chemobrain" -- mental changes such as memory deficits and the inability to focus.
Patients should be advised to make sure that their family and work colleagues understand that just because treatment is over, that doesn't mean that she is going to be able to jump right back into running the office, coaching, and travelling to conferences a week out of every month. The issue of returning to work shortly after the treatment, has been addressed by many organisations, amongst them cancer societies such as the American Cancer Society, who advise that returning to work may help maintain your sense of who you are and how you fit in. However, the return to work should be graded and the possible options should be explored with the employer, like flexi-time, job sharing, or working from home.
Options like these may help the patient ease her mind and body back into the demands of her job. The Association of Chartered Physiotherapists in Occupational Health and Ergonomics referred to their role as:
“Physiotherapists in Occupational Health use their professional knowledge and skills, together with skills for interaction and decision-making/problem-solving to assess the occupational health needs of the workforce, and to design and deliver personalised advice and interventions that maximise an individual’s performance at work”. (ACPOHE 2013)
Vocational Rehabilitation physiotherapists are involved in the early treatment and timely application of appropriate treatment and advice. This allows patients to remain active, suitably return to work following injury, and remain at work upon return [57]. The physiotherapist should develop personalised interventions for each individual patient and duties include assessment of workers abilities, job and task analysis for each individual patient.
The physiotherapist can assist the patient with her plans to return to work by carrying out assessments on the physical capabilities of the patient in relation to the work place. A work place assessment will also benefit the achievement of this goal. Following the workplace assessment, an adjustment of the duties can be recommended to the patient and the employer. The knowledge of anatomy, kinesiology and ergonomics, together with the agreed work place adjustments, will allow the physiotherapist to focus on the treatment of the disease and prevent injuries when the patient returns to work. This will be done by addressing the physical function of the rest of the body which will help the patient maintain independence and confidence. CSP reports, Work Health and Fitness for Work 2014, highlight the role physiotherapy plays in patient’s physical and mental wellbeing and the effectiveness in reducing costs from the sickness absence through improving function and independence by providing advice to the patients and collaborating with the government and other health agencies to support patients to engage with work and influence employers in the decision making of the tasks allocation and management. Fitness Profits is a CSP leaflet which outlines the role of the physiotherapy and the benefits it would have for the patient.
Breast Cancer Recurrence[edit | edit source]
Patients need to be educated to know their bodies and signs and symptoms so if there is recurrence, patients can identify the signs and address this at an early stage. Clinicians should be educated on the potential breast cancer recurrence and have strategies in place to address a reoccurrence for each individual patient. The clinician are expected to know where to turn to within the MDT to treat any issues effectively. The sings and symptoms for reassurance of breast cancer varies between patients and the specific kind of breast cancer, there for clinicians need to undertake specific learning for their patient groups for these.
“Cancer control requires a multilevel strategy that intervenes at different stages of the disease” [58]
Outcome Measures[edit | edit source]
|
Lymphodema |
References[edit | edit source]
- ↑ Eyigor S, Kanyilmaz S. Exercise in patients coping with breast cancer: An overview. World J Clin Oncol 2014 08/10;5(3):406-411.
- ↑ Pidlyskyj K, Roddam H, Rawlinson G, Selfe J. Exploring aspects of physiotherapy care valued by breast cancer patients. Physiotherapy 2014;100:156-161.
- ↑ 3.0 3.1 Cancer Research UK. Cancer Statistics. Cancer of the Breast. :11 November 2014.
- ↑ Langhorne ME, Fulton JS, Otto SE. Oncology nursing. : St. Louis, Mo. : Mosby Elsevier, c2007; 5th ed. / edited by] Martha E. Langhorne, Janet S. Fulton, Shirley E. Otto; 2007.
- ↑ 5.0 5.1 EBAUGH, D., SPINELLI, B. AND SCHMITZ, K.H., 2011. “Shoulder impairments and their association with symptomatic rotator cuff disease in breast cancer survivors”, Medical Hypotheses. Vol. 77, pp. 481–487.
- ↑ 6.0 6.1 6.2 6.3 6.4 6.5 Pacurar R, Miclaus C, Miclaus M. Morbidity associated with breast cancer therapy and the place of physiotherapy in its management. Timisoara Physical Education & Rehabilitation Journal 2011 05;3(6):46-54.
- ↑ JOHNSON, S. and MUSA, I., 2004.Preparation of the breast cancer patient for radiotherapy planning. Physiotherapy. . Vol. 90, no. 4, pp. 195-203.
- ↑ Dahl AA, Nesvold I, Reinertsen KV, Fosså SD. Original Article: Arm/shoulder problems and insomnia symptoms in breast cancer survivors: Cross-sectional, controlled and longitudinal observations. Sleep Med 2011;12:584-590.
- ↑ Freitas-Silva R, de Freitas-Júnior, R. ( 1 ), Conde DM(2), Martinez EZ(3). Comparison of quality of life, satisfaction with surgery and shoulder-arm morbidity in breast cancer survivors submitted to breast-conserving therapy or mastectomy followed by immediate breast reconstruction. Clinics 2010 / 06 / 01 /;65(8):781-787.
- ↑ Harrington S(1), Padua D(2), Myers J(2), Battaglini C(3), Groff D(3), Michener LA(4), et al. Comparison of shoulder flexibility, strength, and function between breast cancer survivors and healthy participants. Journal of Cancer Survivorship 2011 / 06 / 01 /;5(2):167-174.
- ↑ Todd J, Topping A. A survey of written information on the use of post-operative exercises after breast cancer surgery. Physiotherapy 2005;91:87-93.
- ↑ ) Amaral MTPd, de Oliveira M,Maia Freire, Ferreira NdO, Guimarães R, Vidigal, Sarian L, Otávio, Gurgel MSC. Manual therapy associated with upper limb exercises vs. exercises alone for shoulder rehabilitation in postoperative breast cancer. PHYSIOTHER THEORY PRACT 2012 05;28(4):299-306.
- ↑ SAINI, K.S., TAYLOR, C., RAMIREZ, A.J., PALMIERI, C., GUNNARSSON, U., SCHMOLL, H.J., DOLCI, S.M., GHENNE, C., METZGER-FILHO, O., SKRZYPSKI, M., PAESMANS, M., AMEYE, L., PICCART-GEBHART, M.J. and DE AZAMBUJA, E., 2012. Role of the multidisciplinary team in breast cancer management: results from a large international survey involving 39 countries. Annals of Oncology : Official Journal of the European Society for Medical Oncology / ESMO. Apr, vol. 23, no. 4, pp. 853-859.
- ↑ Fleissig A, Jenkins V, Catt S, Fallowfield L. Review: Multidisciplinary teams in cancer care: are they effective in the UK? Lancet Oncology 2006;7:935-943.
- ↑ FISHER, B., ANDERSON, S., BRYANT, J., MARGOLESE, R.G., DEUTSCH, M., FISHER, E.R., JEONG, J. and WOLMARK, N., 2002. Twenty-year follow-up of a randomized trial comparing total mastectomy, lumpectomy, and lumpectomy plus irradiation for the treatment of invasive breast cancer. New England Journal of Medicine. . Vol. 347, no. 16, pp. 1233-1241.
- ↑ 16.0 16.1 Beurskens CHG(1), van Uden, C.J.T. ( 1,2 ), Strobbe LJA(3), Oostendorp, R.A.B. ( 4,5 ), Wobbes T(6). The efficacy of physiotherapy upon shoulder function following axillary dissection in breast cancer, a randomized controlled study. BMC Cancer 2007 / 08 / 30 /;7.
- ↑ Johansen S, Foss K, Nesvold I, L., Malinen E, Foss S, D. Arm and shoulder morbidity following surgery and radiotherapy for breast cancer. Acta Oncol 2014 04;53(4):521-529.
- ↑ Schnur JB(1), Montgomery GH(1), Ouellette SC(2), Dilorenzo TA(3), Green S(4). A qualitative analysis of acute skin toxicity among breast cancer radiotherapy patients. Psychooncology 2011 / 03 / 01 /;20(3):260-268.
- ↑ Cox JD, Stetz J, Pajak TF. Toxicity criteria of the Radiation Therapy Oncology Group (RTOG) and the European Organization for Research and Treatment of Cancer (EORTC). Int J Radiat Oncol Biol Phys 1995 03/30;31(5):1341-1346.
- ↑ Yarnold J. Early and locally advanced breast cancer: diagnosis and treatment National Institute for Health and Clinical Excellence guideline 2009. Clin Oncol (R Coll Radiol) 2009 04;21(3):159-160.
- ↑ HICKEY, B.E., FRANCIS, D.P. and LEHMAN, M., 2013. Sequencing of chemotherapy and radiotherapy for early breast cancer. Cochrane Database Syst Rev. , vol. 4.
- ↑ Monsuez J, Charniot J, Vignat N, Artigou J. Review: Cardiac side-effects of cancer chemotherapy. Int J Cardiol 2010;144:3-15.
- ↑ Senkus E, Jassem J. Complications of Treatment: Cardiovascular effects of systemic cancer treatment. Cancer Treat Rev 2011;37:300-311.
- ↑ Yeh E. Cardiotoxicity induced by chemotherapy and antibody therapy. Annu Rev Med 2006;57:485-498.
- ↑ McAnaw MB, Harris KW. The Role of Physical Therapy in the Rehabilitation of Patients with Mastectomy and Breast Reconstruction. Breast Disease 2002 12;16(1):163-174.
- ↑ Levangie PK, Drouin J. Magnitude of late effects of breast cancer treatments on shoulder function: a systematic review. Breast Cancer Res Treat 2009;116(1):1-15.
- ↑ Torres Lacomba M, Yuste Sánchez MJ, Zapico Goñi A, Prieto Merino D, Mayoral dM, Cerezo Téllez E, et al. Effectiveness of early physiotherapy to prevent lymphoedema after surgery for breast cancer: randomised, single blinded, clinical trial. BMJ (Clinical research ed ) 2010 / 01 / 01 /;340:b5396.
- ↑ 28.0 28.1 28.2 Courneya KS. Exercise in cancer survivors: an overview of research. Medicine & Science in Sports & Exercise 2003 11;35(11):1846-1852.
- ↑ Courneya KS, Friedenreich CM. Physical Activity and Cancer [electronic resource]. : Berlin, Heidelberg : Springer Berlin Heidelberg : Imprint: Springer, 2011; 2011.
- ↑ 30.0 30.1 30.2 PANEL E. American College of Sports Medicine roundtable on exercise guidelines for cancer survivors. 2010.
- ↑ Miedema B, Easley J. Barriers to rehabilitative care for young breast cancer survivors: a qualitative understanding. Supportive Care in Cancer 2012;20(6):1193-1201.
- ↑ Leach H, Danyluk J, Culos–Reed S. Design and implementation of a community-based exercise program for breast cancer patients. Current Oncology 2014;21(5):267.
- ↑ Ottenbacher AJ(1), Day RS(1), Taylor WC(1), Sharma SV(1), Sloane R(2), Snyder DC(3), et al. Exercise among breast and prostate cancer survivors-what are their barriers? Journal of Cancer Survivorship 2011 / 12 / 01 /;5(4):413-419
- ↑ Blanchard CM, Courneya KS, Stein K. Cancer survivors' adherence to lifestyle behavior recommendations and associations with health-related quality of life: results from the American Cancer Society's SCS-II. J Clin Oncol 2008 2008;26(13):2198-2204.
- ↑ Turner, J. ( 1,4 ), Hayes S(2), Reul-Hirche H. Improving the physical status and quality of life of women treated for breast cancer: A pilot study of a structured exercise intervention. J Surg Oncol 2004 / 06 / 01 /;86(3):141-146.
- ↑ Monninkhof EM, Elias SG, Vlems FA, van dT, Schuit AJ, Voskuil DW, et al. Physical activity and breast cancer: a systematic review. Epidemiology 2007 01;18(1):137-157.
- ↑ 37.0 37.1 37.2 Brunet J, Taran S, Burke S, Sabiston CM. A qualitative exploration of barriers and motivators to physical activity participation in women treated for breast cancer. Disability & Rehabilitation 2013 12/25;35(24):2038-2045.
- ↑ 38.0 38.1 Blaney JM(1), Lowe-Strong A, Gracey JH(1), Rankin-Watt J, Campbell A(3). Cancer survivors' exercise barriers, facilitators and preferences in the context of fatigue, quality of life and physical activity participation: A questionnaire-survey. Psychooncology 2013 / 01 / 01 /;22(1):186-194.
- ↑ 39.0 39.1 HEFFERON, K., MURPHY, H., MCLEOD, J., MUTRIE, N., CAMPBELL, A., 2013. Understanding barriers to exercise implementation 5-year post-breast cancer diagnosis: a large-scale qualitative study. Health Education Research [online]. Vol 28, no. 5, pp. 843-6 [viewed November 2014]. Available from: NCBI.
- ↑ LOH, S. Y., CHEW, S., LEE, S., 2010. Physical Activity and Women with Breast Cancer: Insights from Expert Patients [online]. Vol. 11, pp. 87-4 [viewed November 2014]. Available from: NCBI.
- ↑ 41.0 41.1 41.2 41.3 PARAMANANDAM, V. S., & ROBERTS, D., 2014. Weight training is not harmful for women with breast cancer-related lymphoedema: a systematic review. Journal of Physiotherapy [online]. Vol. 60, no. 3, pp. 136-3 [viewed November 2014]. Available from: NCBI.
- ↑ Emery, C.F. ( 1,2,3 ), Yang H-(1), Peterson LJ(1), Suh S(1), Frierson GM(4). Determinants of physical activity among women treated for breast cancer in a 5-year longitudinal follow-up investigation. Psychooncology 2009 / 01 / 01 /;18(4):377-386.
- ↑ Bower JE, Ganz PA, Desmond KA, Rowland JH, Meyerowitz BE, Belin TR. Fatigue in breast cancer survivors: Occurrence, correlates, and impact on quality of life. Journal of Clinical Oncology 2000 / 02 / 01 /;18(4):743-753.
- ↑ Hayes, S. ( 1,2,4 ), Newman B(1), Cornish B(3). Comparison of methods to diagnose lymphoedema among breast cancer survivors: 6-month follow-up. Breast Cancer Res Treat 2005 / 02 / 01 /;89(3):221-226.
- ↑ Hayes SC, Janda M, Cornish B, Battistutta D, Newman B. Lymphedema after breast cancer: Incidence, risk factors, and effect on upper body function. Journal of Clinical Oncology 2008 / 01 / 01 /;26(21):3536-3542.
- ↑ Cheifetz O, Haley L. Management of secondary lymphedema related to breast cancer. Can Fam Physician 2010 12;56(12):1277-1284.
- ↑ MCKENZIE, D.C., A breast in a Boat - a race against breast cancer. Canadian Medical Assoc J [online]. Vol. 159, no.4, pp. 376-78 [viewed November 2014]. Available from: NCBI.
- ↑ Kwan ML, Cohn JC, Armer JM, Stewart BR, Cormier JN. Exercise in patients with lymphedema: a systematic review of the contemporary literature. JOURNAL OF CANCER SURVIVORSHIP-RESEARCH AND PRACTICE 2011;5(4):320-336.
- ↑ Schmitz KH(1), Troxel AB(1), Lewis-Grant L, Bryan CJ(1), Williams-Smith C, Chittams J(1), et al. Weight lifting for women at risk for breast cancer-related lymphedema: A randomized trial. JAMA - Journal of the American Medical Association 2010 / 12 / 22 /;304(24):2699-2705.
- ↑ Schmitz KH, Ahmed RL, Troxel A, Cheville A, Smith R, Lewis-Grant L, et al. Weight lifting in women with breast-cancer-related lymphedema. N Engl J Med 2009 08/13;361(7):664-673.
- ↑ LOH, S. Y., CHEW, S., LEE, S., 2010. Physical Activity and Women with Breast Cancer: Insights from Expert Patients [online]. Vol. 11, pp. 87-4 [viewed November 2014]. Available from: NCBI.
- ↑ 52.0 52.1 Burnham TR, Wilcox A. Effects of exercise on physiological and psychological variables in cancer survivors. / Effets de l ' activite physique sur les variables physiologiques et psychologiques des personnes en phase de remission d ' un cancer. Medicine & Science in Sports & Exercise 2002 12;34(12):1863-1867.
- ↑ World Health Organisation. The International Classification of Functioning, Disability and Health. 2001; Available at: http://www.who.int/disabilities/world_report/2011/report.pdf. Accessed 15 November, 2014.
- ↑ Halm MA. Clinical evidence review. Relaxation: a self-care healing modality reduces harmful effects of anxiety. Am J Crit Care 2009 03;18(2):169-172.
- ↑ Robotin M, Olver I, Girgis A. When cancer crosses disciplines : a physician's handbook. : London : Imperial College Press, c2010; 2010
- ↑ Unruh, A. and Elvin, N. (2004). In the eye of the dragon: Women's experience of breast caner and the occupation of dragon boat racing. R EVUE CANADIENNE D ’ ERGOTHÉRAPIE, 71(3), pp.138-149.
- ↑ Waddell, G., Burton, A.K and Kendall, N.A.S. Vocational Rehabilitation: What Works, for Whom and When?
- ↑ HIATT RA, RIMER BK. Principles and Applications of Cancer Prevention and Control Interventions. Cancer Epidemiology and Prevention 2006.
- ↑ Keeley V, Crooks S, Locke J, Veigas D, Riches K, Hilliam R. A quality of life measure for limb lymphoedema (LYMQOL). J LYMPHOEDEMA 2010 04;5(1):26-37.
- ↑ Mendoza TR(1), Wang XS(1), Cleeland, C.S. ( 1,4 ), Morrissey M(1), Johnson BA(1), Wendt JK(1), et al. The rapid assessment of fatigue severity in cancer patients: Use of the brief fatigue inventory. Cancer 1999 / 01 / 01 /;85(5):1186-1196.
- ↑ Yellen, S.B. ( 1,3 ), Cella DF(1), Webster K(1), Blendowski C(1), Kaplan E(2). Measuring fatigue and other anemia-related symptoms with the Functional Assessment of Cancer Therapy (FACT) measurement system. J Pain Symptom Manage 1997 / 02 / 01 /;13(2):63-74.
- ↑ Hudak, P.L. ( 1,2 ), Amadio, P.C. ( 1,3 ), Bombardier, C. ( 2,4 ). Development of an upper extremity outcome measure: The DASH (disabilities of the arm, shoulder, and head). Am J Ind Med 1996 / 01 / 01 /;29(6):602-608
- ↑ Zigmond AS, Snaith RP. The Hospital Anxiety and Depression Scale. Acta Psychiatr Scand 1983 06;67(6):361.