Pharmacological Management of Rheumatoid Arthritis
Introduction[edit | edit source]
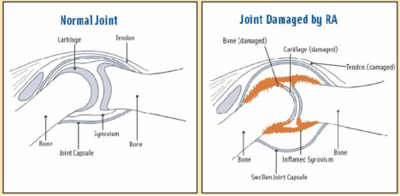
Rheumatoid arthritis (RA) is a chronic, autoimmune disease marked by systemic inflammation of both articular joints and extra-articular areas (e.g. cardiopulmonary systems). See links for more informative on RA
Physical therapy plays a large role in the management of RA and physical therapists (PT) should be aware of the pathophysiology of the disease and the implications of therapeutic interventions used to delay or arrest the progression to provide proper patient care.
Types of Drug Therapies[edit | edit source]
Recently, there have been incredible expansions in the management of RA due to an increasing number of available drug options This brief video gives an overview of drug options
There are two primary sub-classifications of drugs used in the treatment of RA;
- Drugs that provide disease-modifying therapy (DMARDs) DMARDs are medications taken regularly for longer periods of time independent of acute symptoms. DMARDs consist of Traditional DMARDs (DMARDs) and Biological DMARDs (bDMARDs).
- Drugs for symptomatic treatment. DMARDs are medications taken regularly for longer periods of time independent of acute symptoms. Symptomatic therapy is used to relieve acute pain and inflammation associated with the disease process. Non-steroidal anti-inflammatory drugs (NSAIDs), acetaminophen, and corticosteroids are the primary drugs of choice for symptomatic therapy.[2]
Drug Classes
Summary[edit | edit source]
Rheumatoid Arthritis is a complex and ever-changing disease with treatment options that are just as intricate. Treatment regimen is based on disease severity, location of injury, comorbidities or contraindications, cost of drug, and the need for monotherapy or a combination of drugs.[3] The PT must be aware of the effects of each drug category in order to monitor for injurious signs and symptoms the patient may present.
NSAIDs and corticosteroids are recommended for use in the initial stages of RA for short-term and symptomatic pain relief. There are minimal side effects of NSAIDs, but signs and symptoms that may present include GI bleeding, cardiovascular issues, and dizziness.[4][5][6] Corticosteroids are also used in the initial stage as a means of reducing disease activity in patients who are awaiting a response to DMARD therapy.[7] Typical adverse effects of Corticosteroids include immunosuppression, which oftentimes leads to infection, the development of osteoporosis, and other metabolic conditions.[8]
DMARDs and bDMARDs are similar in that they can adversely affect the GI system, pulmonary function, blood pressure, and cause skin irritation.[7]
Although these effects may be seemingly minor in comparison to more malignant conditions, the PT should monitor and report these symptoms as appropriate. Many of these medications may cause the patient to become frail in stature, so the PT must exercise caution during the therapy session. In addition, patient education is a significant component of care and the clinician is responsible for providing relevant treatment while remaining within the physical therapy scope of practice.
References[edit | edit source]
- ↑ Mayo Clinic Drugs for RA Available from: https://www.youtube.com/watch?v=8vu6aNmAano&feature=youtu.be (last accessed 29.12.2019)
- ↑ Singh JA, Saag KG, Bridges SL, et al. 2015 American College of Rheumatology Guideline for the Treatment of Rheumatoid Arthritis. Arthritis Care Res. 2016;68(1):1-25. doi:10.1002/acr.22783.
- ↑ Benjamin O, Lappin SL. Disease Modifying Anti-Rheumatic Drugs (DMARD) [Updated 2018 Oct 27]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2018. Available from: https://www.ncbi.nlm.nih.gov/books/NBK507863/
- ↑ U.S. Food and Drug Administration. Motrin® Ibuprofen Tablets, USP. https://www.accessdata.fda.gov/drugsatfda_docs/label/2007/017463s105lbl.pdf. Last Modified January 20, 2007. Accessed November 21, 2018.
- ↑ U.S. Food and Drug Administration. Voltaren® (diclofenac sodium enteric-coated tablets). https://www.accessdata.fda.gov/drugsatfda_docs/label/2016/019201s046lbl.pdf. Last Modified January 20, 2007. Accessed November 21, 2018.
- ↑ U.S. Food and Drug Administration. Celebrex® celecoxib capsules. https://www.accessdata.fda.gov/drugsatfda_docs/label/2008/020998s026lbl.pdf. Last Modified January 20, 2007. Accessed November 21, 2018.
- ↑ 7.0 7.1 Kumar P, Banik S. Pharmacotherapy options in rheumatoid arthritis. Clin Med Insights Arthritis Musculoskelet Disord. 2013;6:35-43. doi:10.4137/CMAMD.S5558
- ↑ Bingham, C. John Hopkins University. Rheumatoid arthritis treatment. https://www.hopkinsarthritis.org/arthritis-info/rheumatoid-arthritis/ra-treatment/. Accessed October 3, 2018.