Pain Facilitation and Inhibition
Original Editor - Manisha Shrestha
Top Contributors - Manisha Shrestha, Lucinda hampton, Jo Etherton, Kim Jackson, Lauren Lopez, Tolulope Adeniji and Kirenga Bamurange Liliane
Objectives[edit | edit source]
- Define and describe the differences between facilitatory and inhibitory pathways, including their brain sites and neurotransmitters
- Explain and describe how these pathways can be activated by non-pharmacological agents.
Ascending pain pathway[edit | edit source]
Ascending pain pathway is the pathway with afferent fibres. Lateral spinothalamic tract is the ascending tract which carry pain from pheriphery to central. Free nerve endings at tissue level are triggered by inflammatory mediators (Cytokines such as IL-1b, IL-6 and TNF ,prostaglandins) from immune cells of peripheral tissues after any injury. Aδ afferent fibers,which transmit impulses of fast pain secrete glutamate.The C type fibers, which transmit impulses of slow pain secrete substance P. Glutamate and Sustance p are two neurotrasmiters which help to transmit impluse from nerve endings i.e 1st order neuron to 2nd order neuron. 2nd order neuron run from dorsal horn of spinal cord (sustania gelatinosa) to opposite thalamus . The dorsal horn of the spinal cord is the location of the first synapse in pain pathways, and as such, offers a very powerful target for regulation of nociceptive transmission by both local segmental and supraspinal mechanisms. [2]Then 3rd order neuron from thalamus to cortex (primary and secondary somatosensory cortex (S1 and S2 respectively), anterior- and mid-cingulate cortex (ACC and MCC, respectively) and insula).[3]
Descending pain pathway[edit | edit source]
Supraspinal (or descending) pain control pathways arises from a number of supraspinal sites. Descending pain control pathways can be both facilitatory as well as inhibitory. Facilitatory pathways are the one which enhances pain perception where as inhibitory pathways suppresses pain perception. The balance between inhibition and facilitation is dynamic, and can be altered in different behavioral, emotional, psychological and pathological states.[3] Descending pain control pathways plays a critical role in determining the experience of both acute and chronic pain.[2] Facilitatory and inhibitory pathways are further described below.
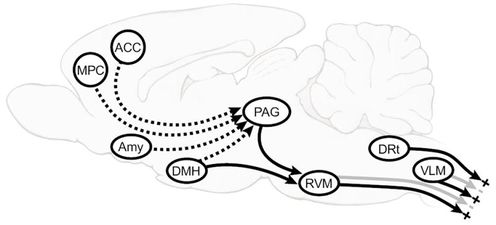
Facilitatory and inhibitory pathways[edit | edit source]
Based on supraspinal sites:[edit | edit source]
- Periacqeductal gray aand Rostroventromedial medulla (PAG_RVM) system
Peri-acqueductal gray in mid brain is heavily interconnected with frontal lobe of cerebral cortex, the hypothalamus and limbic forebrain structures including the amygdala. The PAG projects to the RVM, which in turn sends its output to dorsal horn laminae important in nociceptive function. Rostroventromedial medulla (RVM) play important roles in descending modulation of nociception, which can result either in inhibition and/or facilitation of nociceptive and non-nociceptive inputs. Descending projections from the RVM extend to spinal cord dorsal horns where they connect to primary afferent terminals, second- and third-order neurons, as well as interneurons and thus inturn either inhibit or facilitate the pain sensation. The descending inhibitory function of the RVM is associated with OFF-cell activity, which can be significantly upregulated by endogenous opioids like enkephalin and endorphins.
The amygdala is known to be a critical relay to the PAG-RVM system in the analgesic states associated with intense fear and opioid action in the basolateral nucleus of the amygdala recruits OFF-cells in the RVM. This system has a pivotal role in organising strategies for coping with intrinsic and extrinsic stressors, and is also recognized as the central site of action of analgesic agents including opioids, cyclooxygenase inhibitors, and cannabinoids.
Medial preoptic area is a primary site at which Prostaglandin E2 (PGE2) acts to organize autonomic, neuroendocrine and behavioral elements of the sickness response.Activation of the dorsomedial nucleus of the hypothalamus, a region implicated in autonomic aspects of the response to psychological stress, also evokes behavioral hyperalgesia mediated by ON-cells.
2. Two areas of the caudal medulla, the dorsal reticular nucleus (DRt) and caudal lateral ventrolateral medulla (VLM) also plays important role in pain modulation. VLM potently inhibits behavioral nociceptive responses. DRt facilitates behavioral measures of nociception.[2]
Based on Neurotransmitters:[3][edit | edit source]
Opioid pathway[edit | edit source]
Descending projections from the RVM extend to spinal cord dorsal horns where they connect to primary afferent terminals, second- and third-order neurons, as well as interneurons. Descending inhibitory function of the RVM is associated with OFF-cell activity, which can be significantly upregulated by endogenous opioids. Neuroimaging studies have demonstrated that placebo analgesia is dependent on activation of pain inhibitory systems from cortical and subcortical areas, including the rostral ACC and PAG. Increased activation of these brain areas seems to be associated with placebo analgesia.
Serotonergic pathway[edit | edit source]
Serotonin (5-HT) and norepinephrine, are involved in endogenous pain modulation. Norepinephrine and 5-HT can be released via descending pain pathways to modulate nociceptive signaling in the spinal cord. Norepinephrine inhibits pain through α2 adrenoceptors, while 5-HT seems to have pain facilitatory and inhibitory functions.RVM neurons that have glycinergic or GABAergic projections to the spinal cord to mediate antinociception.
Depending on the receptor subtype, spinal 5-HT can have inhibitory or facilitatory effects on pain. For example, spinal blockade of inhibitory 5-HT receptors abolished the antinociceptive effect of morphine injections into the RVM, while blockade of pain facilitatory 5-HT receptors prevented hyperalgesia.
Noradrenergic pathways[edit | edit source]
Direct stimulation of PAG or RVM does not only increase 5-HT but also norepinephrine concentrations in the cerebrospinal fluid, resulting in pain reductions[3] Although neither PAG nor RVM contain noradrenergic neurons, both regions communicate with norad - renergic brain stem nuclei associated with pain modulation, including the locus coeruleus. These nuclei have noradrenergic projections to the spinal cord, which can inhibit the response of dorsal horn pain transmission neurons.Dorsal horn neuron recordings have shown that activated a2-adrenergic receptors hyperpolarize presynaptic neurons and decrease the release of excitatory neu - rotransmitters from primary afferent terminals, resulting in pain inhibition.
Non-pharmacological agents affecting pain modulation[edit | edit source]
Non- pharmacological agents are the other agents than that of medication which affect the pain modulation systems.
Nonpharmacological approaches to the relief of pain are
- psychological interventions (including distraction, stress management, hypnosis, and other cognitive-behavioral interventions),
- acupuncture and acupressure,
- transcutaneous electrical nerve stimulation,
- physical therapies (including massage, heat/cold, physiotherapy, osteopathy, and chiropractic).[5]
Accumulating evidence supports the important role of supraspinal pain modulation for both analgesia and hyperalgesia. Multiple cortical and subcortical brain and brainstem regions integrate and process sensory, autonomic and emotional information, resulting in activation of the PAG and RVM, with subsequent inhibition or facilitation of pain-related dorsal horn neurons. This top–down modulation is relevant for experimental, as well as clinical pain. These pain modulatory pathways are affected by memories and mood, as well as sociocultural background as different cortical regions like amygdala, hypothalamus are involved in the descending pain modulation pathways.[6]
Nonpharmacological analgesia therefore involves the inhibition of nociceptive input by activating separate antinociceptive outputs. Electroacupuncture has been shown to enhance the expression of serotonin and reduce the release of substance P during electroacupuncture inhibition of acute nociceptive responses.TENS appears to produce both segmental and descending pain inhibition since inhibition remains after spinalization (removal of descending inhibition) and concentrations of endogenous opioids have been shown to increase in cerebrospinal fluid following TENS procedure.[5]
References[edit | edit source]
References will automatically be added here, see adding references tutorial.
- ↑ Armando Hasudungan. PAIN! Physiology - The Ascending Pathway, Descending Pain Pathway and the Substantia Gelatinosa. Available From: https://www.youtube.com/watch?v=5c8maFAhqIc [last accessed 22/2/2020]
- ↑ 2.0 2.1 2.2 Heinricher MM, Tavares I, Leith JL, Lumb BM. Descending control of nociception: specificity, recruitment and plasticity. Brain research reviews. 2009 Apr 1;60(1):214-25.
- ↑ 3.0 3.1 3.2 3.3 Staud R. The important role of CNS facilitation and inhibition for chronic pain. International journal of clinical rheumatology. 2013 Dec 1;8(6):639.
- ↑ Heinricher MM, Tavares I, Leith JL, Lumb BM. Descending control of nociception: specificity, recruitment and plasticity. Brain research reviews. 2009 Apr 1;60(1):214-25.
- ↑ 5.0 5.1 Pak SC, Micalos PS, Maria SJ, Lord B. Nonpharmacological interventions for pain management in paramedicine and the emergency setting: a review of the literature. Evidence-Based Complementary and Alternative Medicine. 2015;2015.
- ↑ Zhuo M. Descending facilitation: From basic science to the treatment of chronic pain. Molecular pain. 2017 Mar;13:1744806917699212.






