Multidimensional Nature of Pain
Original Editor - Alberto Bertaggia.
Top Contributors - Alberto Bertaggia, Nina Myburg, Admin, Kim Jackson, Michelle Lee, Vidya Acharya, 127.0.0.1, WikiSysop, Lauren Lopez, Jess Bell and Jo Etherton
Introduction[edit | edit source]
A definition of pain is provided by the International association for the Study of Pain (IASP) as follows[1]:
"An unpleasant sensory and emotional experience associated with actual or potential tissue damage, or described in terms of such damage"
Pain is always subjective and everyone learns the use of this word through experiences related to injury in early life.
It is a sensation in a part or parts of the body, but it is also always unpleasant and therefore also emotional.
Even in the absence of tissue damage or any likely pathophysiological cause, people still report pain; usually this happens for psychological reasons. In these cases, it is challenging to distinguish whether their experience arise from a damaged tissue or not, based only upon the subjective report[2].
It is important to underline that activity induced in the nociceptor and nociceptive pathways by a noxious stimulus is not pain[2], which is always the output of a widely distributed neural network in the brain rather than one coming directly by sensory input evoked by injury, inflammation, or other pathology[3].
In the following video Karen D. Davis tries to explain why do some people react to the same painful stimulus in different ways.
Relevant anatomy and physiology[edit | edit source]
Nociceptors[edit | edit source]
Nociceptors (from the latin nocere = to hurt) are sensory receptors which detect signals from damaged tissue or the threat of damage and indirectly also respond to chemicals released from the damaged tissue. There are free nerve endings present in many types of tissues, and cell bodies located in the dorsal root ganglions or in the cranial nerve ganglia.
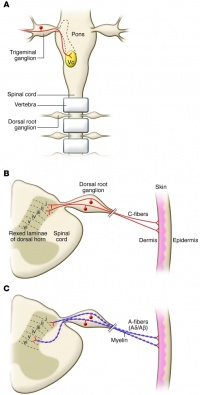
Nociceptors have unmyelinated (C-fiber) or thinly myelinated (A-fiber) axons[4]. C-fibers support conduction velocities of 0.4–1.4 m/s, while A-fibers support conduction velocities of approximately 5–30 m/s[5].
Nociception
[edit | edit source]
Nociception is a mechanism which comprises the processes of transduction, conduction, transmission and perception[6].
- Transduction is the conversion of a noxious thermal, mechanical, or chemical stimulus into electrical activity in the peripheral terminals of nociceptor sensory fibers. This process is mediated by specific receptor ion channels expressed only by nociceptors.
- Conduction is the passage of action potentials from the peripheral terminal along axons to the central terminal of nociceptors in the central nervous system.
- Transmission is the synaptic transfer and modulation of input from one neuron to another.
- Projection neurons in the dorsal horn transfer nociceptive input to the brainstem, hypothalamus, and thalamus and then, through relay neurons, to the cortex. Here is where perception occur as a subjective experience.
Types of pain classification[edit | edit source]
Based upon the works of Woolf[6][7], this is a useful types of pain classification:
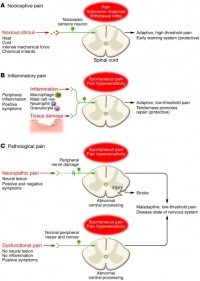
- Nociceptive pain. This kind of pain is concerned with the sensing of noxious stimuli and is a high-threshold pain only activated in the presence of intense stimuli. It has a protective role requiring immediate attention and responses (i.e. withdrawal reflex).
- Inflammatory pain. This second kind of pain is important to promote protection and healing of the injured tissues by creating an enviroment which suggests avoidance of movement and stress of the body parts. This is made possible by activation of the immune system causing inflammation.
- Pathological pain. This type of pain is uncoupled from noxious stimulii and even from tissue damage, it is not protective, and results from abnormal functioning of the nervous system (peripheral or central). To note, this is a low-threshold pain. This time pain is not a symptom, but rather a disease itself. It occurs with peripheral sensitization and central sensitization.
Acute and chronic pain
[edit | edit source]
Acute pain is caused by a noxious stimuli ad is mediated by nociception. It has early onset and serve to prevent tissues damages. It is also useful to learn to avoid threat of damage, because certain categories of noxious stimulii become linked to the sensation of pain. This is why this type of pain is defined as adaptive, it helps to survive and to heal[6]
Chronic pain is pain continuing beyond 3 months or after healing is complete[2]. It may arise as a consequence of tissue damage or inflammation or have no identified cause[8]. It can affect a specific body part (i.e. Complex Regional Pain Syndrome (CRPS), low back pain (LBP), pelvic pain) or be widespread (i.e. fibromyalgia). Chronic pain is a complex condition embracing physical, social and psychological factors, consequently leading to disability, loss of independence and poor quality of life (QoL)[9].
Psychological factors in pain
[edit | edit source]
Anxiety
[edit | edit source]
Health anxious individuals form dysfunctional assumptions and beliefs about symptoms and disease based on past experiences and become health anxious when these dysfunctional scheme are triggered by critical incidents[10]. Moreover, they will have a tendency to misinterpret somatic information as catastrophic and personally threatening[10].
Some studies report an increase in pain correlated with increased levels of anxiety, but other suggests it has no effects, thus the effect of anxiety on pain may be dependent on attention[11].
Clinically, anxiety can compromise treatments as the practiotioners must expect patients to report catastrophization and greater pain during activities, thus there is need to target attentional focus and interpretation of sensations among health anxious clients[10].
Depression
[edit | edit source]
There are strong evidencies of an established comorbidity of pain and depression[12][13]. Furthermore, when patients with pain have comorbid depression, they have greater pain, a worse prognosis, and more functional disability[14]. Additionally, chronic pain patients with co-morbid depression have higher health care costs compared to pain patients who do not have depression[15].
Pain and depression are associated by neurobiological, cognitive, affective and behavioral factors, thus the optimal treatment approach for comorbid pain and depression should simultaneously address both physical and psychological symptoms[16].
Expectation
[edit | edit source]
Pain perceived when expected may vary based upon the types of cues received (i.e. cues indicates a more intense or damaging stimulus, then more intense pain is perceived, and viceversa) and even cues of an impending decrease in pain, for example the process of taking an analgesic, usually decrease pain[11]. Thus, expectation is thought to play a major role in placebo analgesia[17][18].
Attention and distraction
[edit | edit source]
There is strong evidence that attention (and distraction) is highly effective in modulating the pain experience and demonstrate how cognitive processes can interfere with pain perception[19][20][21][22][23][24][25]. When a person is distracted with a cognitive task pain is perceived as less intense[19][22][26][27][28], even in chronic pain patients[29]. Conversely, pain increases when the pain is in the focus of attention[30]. Functional brain imaging and neurophysiological studies have shown that attention- and cognitive distraction-related modulations of nociceptive-driven activations take place in various pain-sensitive cortical und subcortical brain regions, accompanied by concordant changes in pain perception[19][22][23][24][26][28][31]. At present time, there are various hypothesis on the physiological bases of these phenomenons, although it is likely that a top-down modulation occur[32][33]. Previous studies on pain processing have demonstrated that key regions of the descending pain control system show enhanced responses during attentional distraction[19][22][26][25].
Fear[edit | edit source]
Pain-related fear is a general term to describe several forms of fear with respect to pain[34]. Fear of pain can be directed toward the occurrence or continuation of pain, toward physical activity, or toward the induction of (re)injury or physical harm[35].
Fear toward physical activity is also know as kinesiophobia, and is defined as “an excessive, irrational, and debilitating fear of physical movement and activity resulting from a feeling of vulnerability to painful injury or re-injury”[36][37].
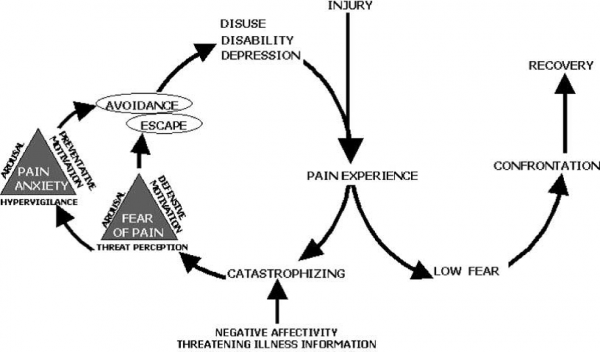
If pain, possibly caused by an injury, is interpreted as threatening (pain catastrophizing), pain-related fear evolves leading to avoidance behaviors, and hypervigilance to bodily sensations followed by disability, disuse and depression. This will maintain the pain experiences thereby fueling the vicious circle of increasing fear and avoidance[38].
In non-catastrophizing patients, no pain-related fear and rapid confrontation with daily activities is likely to occur, leading to fast recovery[38].
These concepts are explained by the Fear-Avoidance (FA) model, which was largely hypothetical in the beginning, but currently there is ample evidence to support the validity of the original FA model[39].
As of today, the FA model is considered to be a component in the development of disability in a variety of conditions, such as low back pain, chronic headache, whiplash disorder, osteoarthritis, knee injury pain, chronic-fatigue syndrome, fibromyalgia and neuropathic pain [40][41][42]
Social and cultural factors in pain
[edit | edit source]
Culture is defined as "the beliefs, customs, arts, etc., of a particular society, group, place, or time" or "a particular society that has its own beliefs, ways of life, art, etc."[43].
Culturally-specific attitudes and beliefs about pain can influence the manner in which individuals view and respond both to their own pain and to the pain of others[44]. Cultural factors related to the pain experience include pain expression, pain language, lay remedies for pain, social roles, and expectations and perceptions of the medical care system[44].
Race/ethnicity, by virtue of their culturally-specific attitudes and beliefs, seems to have an impact on pain processing, including emotional and behavioural responses associated with chronic pain, larger in later stages[45][46].
Another psychosocial factor that may influence differences in pain sensitivity response is the gender role: individuals who considered themselves more masculine and less sensitive to pain have been shown to have higher pain thresholds and tolerances[47].
Socioeconomic factors (e.g. lower levels of education and income) seems to be correlated with a higher incidence of chronic pain diagnosis[48] and pain perception level[49].
The biopsychosocial model of pain
[edit | edit source]
In the past, psychological and physiological (or patophysiological) factors were considered as separated components in a dualistic point of view. Later, the recognition that psychosocial factors, such as emotional stress, could impact the reporting of symptoms, medical disorders, and response to treatment lead to the development of the biopsychosocial model of pain[50].
The bio part is rapresented by the pathophysiology of the disease, or the mechanism of injury, and the relative nociception processes, considering the physiological aspects.
The psychosocial factors (as explaned above) involve both emotion (the more immediate reaction to nociception and is more midbrain based) and cognition (which attach meaning to the emotional experience)[50]. These could trigger additional emotional reactions and thereby amplify the experience of pain, thus perpetuating a vicious circle of nociception, pain, distress, and disability[50].
As of today there are evidencies of psychological factors, such as fear and anxiety, play an important role in the development of chronic pain[51].
Clinical implications
[edit | edit source]
It has to be understood that there is an interaction among physiologic, psychological, and social factors[11], which perpetuates and may even worsen the clinical presentations[50].
- There is the need to have sound knowledge of these interaction mechanism[11].
- Targeting psychosocial factors should be a key component of physiotherapist-led intervention[52].
- Treatment programs must be individually-tailored in order to specifically address the patients' attitudes and beliefs to improve treatment adherence and outcome[41].
Resources[edit | edit source]
Other Physiopedia Pages[edit | edit source]
Pain Course All Physiopedia pages with PAIN as their category. Psychological approaches to pain management
External links[edit | edit source]
- International Asociation for the Sudy of Pain (IASP) site
- Pain Science site
- Pain-ed site
- Body in mind site
- Pain community centre page on multidimensional nature of pain
Recent Related Research (from Pubmed)[edit | edit source]
References[edit | edit source]
- ↑ IASP Taxonomy - IASP [Internet]. [cited 2016 Mar 18]. Available from: http://www.iasp-pain.org/Taxonomy#Pain
- ↑ 2.0 2.1 2.2 Merskey H, Bogduk N. Classification of Chronic Pain: Descriptions of Chronic Pain Syndromes and Definitions of Pain Terms. IASP Press; 1994. 248 p.
- ↑ Melzack R. Pain and the neuromatrix in the brain. J Dent Educ. 2001 Dec;65(12):1378–82.
- ↑ McCleskey EW, Gold MS. Ion channels of nociception. Annu Rev Physiol. 1999;61:835–56.
- ↑ Dubin AE, Patapoutian A. Nociceptors: the sensors of the pain pathway. J Clin Invest. 2010 Nov 1;120(11):3760–72.
- ↑ 6.0 6.1 6.2 Woolf CJ, American College of Physicians, American Physiological Society. Pain: moving from symptom control toward mechanism-specific pharmacologic management. Ann Intern Med. 2004 Mar 16;140(6):441–51.
- ↑ Woolf CJ. What is this thing called pain? J Clin Invest. 2010 Nov 1;120(11):3742–4.
- ↑ Chronic Pain: Symptoms, Diagnosis, & Treatment | NIH MedlinePlus the Magazine [Internet]. [cited 2016 Mar 28]. Available from: https://www.nlm.nih.gov/medlineplus/magazine/issues/spring11/articles/spring11pg5-6.html
- ↑ Breivik H, Collett B, Ventafridda V, Cohen R, Gallacher D. Survey of chronic pain in Europe: prevalence, impact on daily life, and treatment. Eur J Pain. 2006 May;10(4):287–333.
- ↑ 10.0 10.1 10.2 Hadjistavropoulos HD, Hadjistavropoulos T, Quine A. Health anxiety moderates the effects of distraction versus attention to pain. Behaviour Research and Therapy. 2000 May;38(5):425–38.
- ↑ 11.0 11.1 11.2 11.3 Moseley GL. Reconceptualising pain according to modern pain science. Physical Therapy Reviews. 2007 Sep 1;12(3):169–78.
- ↑ Miller LR, Cano A. Comorbid chronic pain and depression: who is at risk? J Pain. 2009 Jun;10(6):619–27.
- ↑ Bair MJ, Robinson RL, Katon W, Kroenke K. Depression and pain comorbidity: a literature review. Arch Intern Med. 2003 Nov 10;163(20):2433–45.
- ↑ Börsbo B, Peolsson M, Gerdle B. The complex interplay between pain intensity, depression, anxiety and catastrophising with respect to quality of life and disability. Disabil Rehabil. 2009;31(19):1605–13.
- ↑ Baumeister H, Knecht A, Hutter N. Direct and indirect costs in persons with chronic back pain and comorbid mental disorders--a systematic review. J Psychosom Res. 2012 Aug;73(2):79–85.
- ↑ Goesling J, Clauw DJ, Hassett AL. Pain and Depression: An Integrative Review of Neurobiological and Psychological Factors. Curr Psychiatry Rep. 2013 Nov 10;15(12):1–8.
- ↑ Pollo A, Amanzio M, Arslanian A, Casadio C, Maggi G, Benedetti F. Response expectancies in placebo analgesia and their clinical relevance. Pain. 2001 Jul;93(1):77–84.
- ↑ Benedetti F, Pollo A, Lopiano L, Lanotte M, Vighetti S, Rainero I. Conscious expectation and unconscious conditioning in analgesic, motor, and hormonal placebo/nocebo responses. J Neurosci. 2003 May 15;23(10):4315–23.
- ↑ 19.0 19.1 19.2 19.3 Bantick SJ, Wise RG, Ploghaus A, Clare S, Smith SM, Tracey I. Imaging how attention modulates pain in humans using functional MRI. Brain. 2002 Feb;125(Pt 2):310–9.
- ↑ Buhle J, Wager TD. Performance-dependent inhibition of pain by an executive working memory task. Pain. 2010 Apr;149(1):19–26.
- ↑ Eccleston C. Chronic pain and distraction: an experimental investigation into the role of sustained and shifting attention in the processing of chronic persistent pain. Behav Res Ther. 1995 May;33(4):391–405.
- ↑ 22.0 22.1 22.2 22.3 Valet M, Sprenger T, Boecker H, Willoch F, Rummeny E, Conrad B, et al. Distraction modulates connectivity of the cingulo-frontal cortex and the midbrain during pain--an fMRI analysis. Pain. 2004 Jun;109(3):399–408.
- ↑ 23.0 23.1 Villemure C, Bushnell MC. Cognitive modulation of pain: how do attention and emotion influence pain processing? Pain 95, 195-199. Pain. 2002;95(3):195–9.
- ↑ 24.0 24.1 Wiech K, Seymour B, Kalisch R, Stephan KE, Koltzenburg M, Driver J, et al. Modulation of pain processing in hyperalgesia by cognitive demand. Neuroimage. 2005 Aug 1;27(1):59–69.
- ↑ 25.0 25.1 Tracey I, Ploghaus A, Gati JS, Clare S, Smith S, Menon RS, et al. Imaging attentional modulation of pain in the periaqueductal gray in humans. J Neurosci. 2002 Apr 1;22(7):2748–52.
- ↑ 26.0 26.1 26.2 Petrovic P, Petersson KM, Ghatan PH, Stone-Elander S, Ingvar M. Pain-related cerebral activation is altered by a distracting cognitive task. Pain. 2000 Mar;85(1-2):19–30.
- ↑ Bingel U, Rose M, Gläscher J, Büchel C. fMRI reveals how pain modulates visual object processing in the ventral visual stream. Neuron. 2007 Jul 5;55(1):157–67.
- ↑ 28.0 28.1 Frankenstein UN, Richter W, McIntyre MC, Rémy F. Distraction modulates anterior cingulate gyrus activations during the cold pressor test. Neuroimage. 2001 Oct;14(4):827–36.
- ↑ McCracken LM, Turk DC. Behavioral and cognitive-behavioral treatment for chronic pain: outcome, predictors of outcome, and treatment process. Spine. 2002 Nov 15;27(22):2564–73.
- ↑ Quevedo AS, Coghill RC. Attentional modulation of spatial integration of pain: evidence for dynamic spatial tuning. J Neurosci. 2007 Oct 24;27(43):11635–40.
- ↑ Hauck M, Lorenz J, Engel AK. Attention to Painful Stimulation Enhances γ-Band Activity and Synchronization in Human Sensorimotor Cortex. J Neurosci. 2007 Aug 29;27(35):9270–7.
- ↑ Corbetta M, Shulman GL. Control of goal-directed and stimulus-driven attention in the brain. Nat Rev Neurosci. 2002 Mar;3(3):201–15.
- ↑ Gilbert CD, Sigman M. Brain states: top-down influences in sensory processing. Neuron. 2007 Jun 7;54(5):677–96.
- ↑ Gebhart GF, Schmidt RF, editors. Fear of Pain. In: Encyclopedia of Pain [Internet]. Springer Berlin Heidelberg; 2013 [cited 2016 Mar 29]. p. 1267–1267. Available from: http://link.springer.com/referenceworkentry/10.1007/978-3-642-28753-4_200800
- ↑ Helsen K, Leeuw M, Vlaeyen JWS. Fear and Pain. In: Gebhart GF, Schmidt RF, editors. Encyclopedia of Pain [Internet]. Springer Berlin Heidelberg; 2013 [cited 2016 Mar 29]. p. 1261–7. Available from: http://link.springer.com/referenceworkentry/10.1007/978-3-642-28753-4_1482
- ↑ Kori SH, Miller RP, Todd DD. Kinisophobia: A new view of chronic pain behavior. Pain manage. 1990 Jan 1;3(1):35–43.
- ↑ Lundberg M, Larsson M, Ostlund H, Styf J. Kinesiophobia among patients with musculoskeletal pain in primary healthcare. J Rehabil Med. 2006 Jan;38(1):37–43.
- ↑ 38.0 38.1 Vlaeyen JWS, Linton SJ. Fear-avoidance and its consequences in chronic musculoskeletal pain: a state of the art. Pain. 2000 Apr 1;85(3):317–32.
- ↑ Crombez G, Eccleston C, Van Damme S, Vlaeyen JWS, Karoly P. Fear-avoidance model of chronic pain: the next generation. Clin J Pain. 2012 Jul;28(6):475–83.
- ↑ Wertli MM, Rasmussen-Barr E, Weiser S, Bachmann LM, Brunner F. The role of fear avoidance beliefs as a prognostic factor for outcome in patients with nonspecific low back pain: a systematic review. The Spine Journal. 2014 May 1;14(5):816–36.e4.
- ↑ 41.0 41.1 Nijs J, Roussel N, Oosterwijck JV, Kooning MD, Ickmans K, Struyf F, et al. Fear of movement and avoidance behaviour toward physical activity in chronic-fatigue syndrome and fibromyalgia: state of the art and implications for clinical practice. Clin Rheumatol. 2013 May 3;32(8):1121–9.
- ↑ Leeuw M, Goossens MEJB, Linton SJ, Crombez G, Boersma K, Vlaeyen JWS. The fear-avoidance model of musculoskeletal pain: current state of scientific evidence. J Behav Med. 2007 Feb;30(1):77–94.
- ↑ Definition of CULTURE [Internet]. [cited 2016 Mar 31]. Available from: http://www.merriam-webster.com/dictionary/culture
- ↑ 44.0 44.1 Shavers VL, Bakos A, Sheppard VB. Race, ethnicity, and pain among the U.S. adult population. J Health Care Poor Underserved. 2010 Feb;21(1):177–220.
- ↑ Campbell CM, Edwards RR. Ethnic differences in pain and pain management. Pain Manag. 2012 May;2(3):219–30.
- ↑ Riley JL, Wade JB, Myers CD, Sheffield D, Papas RK, Price DD. Racial/ethnic differences in the experience of chronic pain. Pain. 2002 Dec;100(3):291–8.
- ↑ Alabas OA, Tashani OA, Tabasam G, Johnson MI. Gender role affects experimental pain responses: a systematic review with meta-analysis. Eur J Pain. 2012 Oct;16(9):1211–23.
- ↑ Jöud A, Petersson IF, Jordan KP, Löfvendahl S, Grahn B, Englund M. Socioeconomic status and the risk for being diagnosed with spondyloarthritis and chronic pain: a nested case-control study. Rheumatol Int. 2014 Sep;34(9):1291–8.
- ↑ Miljković A, Stipčić A, Braš M, Dorđević V, Brajković L, Hayward C, et al. Is experimentally induced pain associated with socioeconomic status? Do poor people hurt more? Med Sci Monit. 2014;20:1232–8.
- ↑ 50.0 50.1 50.2 50.3 Gatchel RJ, Peng YB, Peters ML, Fuchs PN, Turk DC. The biopsychosocial approach to chronic pain: scientific advances and future directions. Psychol Bull. 2007 Jul;133(4):581–624.
- ↑ Hasenbring MI, Chehadi O, Titze C, Kreddig N. Fear and anxiety in the transition from acute to chronic pain: there is evidence for endurance besides avoidance. Pain Management. 2014 Sep 1;4(5):363–74.
- ↑ Woby SR, Roach NK, Urmston M, Watson PJ. The relation between cognitive factors and levels of pain and disability in chronic low back pain patients presenting for physiotherapy. Eur J Pain. 2007 Nov;11(8):869–77.