Inhalation Injury
Original Editor - Joseph Ayotunde Aderonmu
Top Contributors - Joseph Ayotunde Aderonmu, Chelsea Mclene, Naomi O'Reilly and Kim Jackson
Introduction[edit | edit source]
Inhalation injury is one of the most challenging injuries for burn care providers, since it is one of the classic determinants of mortality from severe burn injury; other determinants include age, extent of injury, and delays of resuscitation.[1] It refers to pulmonary injury resulting from inhalation of smoke or chemical products of combustion.[2]
Inhalation injury results in localized damage through direct cellular damage, alterations to regional blood flow and perfusion, airway obstruction, and toxin and pro-inflammatory cytokine release.[3][4] Inhalation injuries results in incapacitation of mucociliary clearance and impairment of alveolar macrophages.[5] It predisposes patients to bacterial infection, specifically and primarily pneumonia, a leading cause of death for burn patients.[6][7]
Epidemiology[edit | edit source]
Inhalation injury usually accompanies up to one-third of all burn injuries and it accounts to up to 90% of all burn-related mortality.[3][8] Up to 10.3% of burn patients were reported by the National Burn Repository of the American Burn Association to have concomitant inhalation injury.[9] As such, 1 in 10 burn patients surviving to admission will have the inhalation injury with the respective increase in the mortality rate.[10]
Classes[edit | edit source]
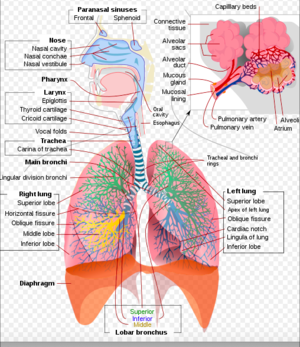
Anatomically, inhalation injuries are divided into three classes:[11]
Heat Injury to the Upper Airway[edit | edit source]
When the temperature of the air in a room containing a fire reaches 1000°F, injury is caused to airway structures above the carina due to the combination of efficient heat dissipation in the upper airway, low heat capacity of air and reflex closure of the larynx.[12]
Injury to these airway structures may cause massive swelling of the tongue, epiglottis, and aryepiglottic folds with obstruction. Airway swelling develops over a matter of hours as fluid resuscitation is ongoing. Initial evaluation is not a good indicator of the severity of obstruction that may occur later.[13]
Chemical Injury to the Lower Airways[edit | edit source]
Generation of materials toxic to the respiratory tract during burning may result in local chemical irritation throughout the respiratory tract.[2] Burning rubber and plastic produces sulfur dioxide, nitrogen dioxide, ammonia and chlorine with strong acids and alkali when combined with water in the airways and alveoli. Laminated furniture contains glues and wall paneling also may release cyanide gas when burned. Burning cotton or wool produces toxic aldehydes. Smoke-related toxins damage epithelial and capillary endothelial cells of the airway.[11][14]
Systemic Toxicity due to Carbon Monoxide or Cyanide Exposure[edit | edit source]
Carbon monoxide poisoning is a major source of early morbidity in burn-injured patients with many fatalities occurring at the scene of the fire due to this mechanism. Carboxyhemoglobin levels exceed 10% in a closed space fire. Significant injury may occur in a short period of time with the exposure with as little as 10% carboxyhemoglobin.[11][15][16]Carbon monoxide competitively inhibits intracellular cytochrome oxidase enzyme systems, most notably cytochrome P-450 resulting in inability of cellular systems to utilize oxygen.[2]
Inhaled hydrogen cyanide, produced during combustion of multiple household materials, also inhibits the cytochrome oxidase system and may have a synergistic effect with carbon monoxide producing tissue hypoxia and acidosis as well as a decrease in cerebral oxygen consumption.[11][16]
Pathophysiology[edit | edit source]
The extent of damage from an inhalation injury depends on the environment and the host: the source of injury, temperature, concentration, and solubility of the toxic gases generated, and the response to that injury by the individual.[17] Inhalation injuries cause formation of casts, reduction of available surfactant, increased airway resistance, and decreased pulmonary compliance[18] leading to acute lung injury and acute respiratory distress syndrome.[19]
The mechanism of destruction can be classified in one of four ways:
Upper Airway Injury
The major pathophysiology seen in the upper airway inhalation injury is induced by microvascular changes from direct thermal injury and chemical irritation.[6]The heat denatures protein, which subsequently activates the complement cascade causing the release of histamine.[17][20] Subsequently, there is the formation of xanthine oxidase, and release of reactive oxygen species (ROS) which combines with nitric oxide in the endothelium to induce upper airway edema area by increasing the microvascular pressure and local permeability[17][21][22] Pro-inflammatory cytokines, ROS and Eicosanoids attract polymorphonuclear cells to the area further amplifying ROS and signaling proteases.[1]
There is a substantial increase in microvascular hydrostatic pressure, a decrease in interstitial hydrostatic pressure and an increase in interstitial oncotic pressure.[17] The hallmark of burn resuscitation is the administration of large amounts of crystalloid, which reduces plasma oncotic pressure affecting the oncotic pressure gradient in the microcirculation causing significantly more airway edema.[17] Barring steam inhalation injuries and blast injuries, the upper airway efficiently protects the lower airway via heat exchange to limit distal damage to the lower airway.[1]
Lower Airway Injury
Injury to the lower airway is due to the chemicals in smoke. The heat capacity of air is low and the bronchial circulation very efficient in warming or cooling the airway gases, so that most gases are at body temperature as they pass the glottis.[23] In order to induce thermal injury to the airway, flames must be in direct contact.[24] Accelerants, or burned biological materials are caustic to the airways and induce an initial response to trigger proinflammatory response. There is a 10-fold increase in bronchial blood flow within minutes of an inhalation injury[16] which is sustained and causes increased permeability and destruction of the bronchial epithelium.[17] There is a subsequent increase in pulmonary transvascular fluid and a fall in PaO2/FiO2 ≤ 200 nearly 24 hours after injury.[25] There is a subsequent hyperemia of the tracheobronchial tree and lower airways and that clinical finding – so prevalent – is used to diagnose the injury.[26][27]Early in the injury, the secretions, from goblet cells, are copious and foamy in nature. In hours to days these secretions solidify forming casts and airway obstruction.[17]
Pulmonary parenchymal injury
Changes to lung parenchyma are delayed, dependent on the severity of injury and the patient’s response to the injury. Parenchymal injuries are associated with an increase in pulmonary transvascular fluid which is directly proportional to the duration of exposure of smoke and toxins.[2] As stated previously, injury to the lower airways and lung parenchyma is rarely due to direct thermal contact. Only steam can overcome the very efficient upper airway heat dissipating capabilities. There is a reduction to the permeability of protein, an increase in the permeability to small particles, an increase in pressure in the pulmonary microvasculature pressure, and a loss of hypoxic pulmonary vasoconstriction.[17] The key pathological derangements in inhalation injury are edema, decreased pulmonary compliance from extravascular lung water and pulmonary lymph, and immediate inactivation of surfactant. There is a subsequent ventilation perfusion mismatch that can lead to profound hypoxemia and ARDS.[6]
Systemic toxicity.
Systemic toxic changes are caused by the inhalation of chemicals and cytotoxic liquids, mists, fumes and gases. Smoke combines with these toxins and increases mortality by increasing tissue hypoxia, metabolic acidosis, and decreasing cerebral oxygen consumption and metabolism.[16][28]
Diagnosis[edit | edit source]
Traditionally, diagnosis of inhalation injury was based on the following indirect observations:[29]
- Facial burns
- Singed nasal vibrissae
- A history of injury in an enclosed space
Taken individually, each of these signs has a high incidence of false positivity, but as a group they have been found to actually underestimate the true incidence of inhalation injury. Carbonaceous secretions represent another classic sign of smoke inhalation that is a less exact predictor of the presence or severity of injury than is popularly believed. Carbonaceous secretions should be regarded as an indicator of exposure to smoke but should not establish either the diagnosis of inhalation injury or its sequela. Hypoxia, rales, rhonchi and wheezes are seldom present on admission, occurring only in those with the most severe injury and implying an extremely poor prognosis.[6]
The admission chest X-ray has also been shown to be a very poor indicator.[6] Although two-thirds of patients develop changes of diffuse or focal infiltrates or pulmonary edema within 5–10 days of injury, the admission film is seldom diagnostic but is important for baseline evaluations.[30]
The current standard for diagnosis of inhalation injury in most major burn centers is fiberoptic bronchoscopy.[31] Useful only in identifying upper airway injury, findings include the presence of soot, charring, mucosal necrosis, airway edema and inflammation.[32]
Bronchoscopy without findings cannot rule out the possibility of parenchyma damage. To evaluate true parenchyma damage, Xenon scanning has been utilized.[33] This is a safe, rapid test requiring a minimum of patient cooperation, it involves serial chest scintiphotograms after an initial intravenous injection of radioactive Xenon gas. It demonstrates areas of the decreased alveolar gas washout, which identifies sites of small airway obstruction caused by edema or fibrin cast formation.[6]
Management[edit | edit source]
There is no consensus amongst leading burn centers the optimal treatment protocol for inhalation injury.[1] The fundamental tenet of treatment for inhalation injury is supportive care through the acute hospitalization and rehabilitation. [1]
Supportive Care[edit | edit source]
Inhalation injuries cause formation of casts, reduction of available surfactant, increased airway resistance, and decreased pulmonary compliance.[18] Patients require pulmonary toilet, physiotherapy, airway suctioning, therapeutic serial bronchoscopies, and early ambulation.[1]
Bronchodilators[edit | edit source]
Bronchodilators decrease airflow resistance and improve airway compliance. β2-adrenergic agonists such as albuterol and salbutamol decrease airway pressure by relaxing smooth muscle and inhibiting bronchospasm thereby increasing the PaO2/FiO2 ratio.[34]
Muscarinic receptor antagonists[edit | edit source]
Muscarinic receptor antagonists such as tiotropium decrease airway pressures and mucus secretion and limit cytokine release by causing smooth muscle constriction within the airways, and stimulation of submucosal glands.[35][36]
Both beta agonists and muscarinic receptor antagonists decrease the host inflammatory response after inhalation injury. Anatomically, there are muscarinic and adrenergic receptors found lining the respiratory tract. How that impacts the inflammatory response and host response is largely unknown. They have been shown to decrease pro-inflammatory cytokines after stress.[37]
Inhaled (nebulized) Mucolytic agents and Anticoagulants[edit | edit source]
The airway obstruction secondary to mucus, fibrin cast formation, and cellular debris subsequent to inhalation injury are addressed by mucolytic agents, specifically, N Acetylcysteine (NAC).[38] NAC is an antioxidant and free radical scavenger with antiinflammatory properties.[39] It is a powerful mucolytic agent that attenuates ROS damage[26]. Inhaled anticoagulants are also used to mitigate airway obstruction from fibrin casts.
Respiratory support[edit | edit source]
There is often such significant upper airway edema from the inhalation injury, or the resuscitation of the cutaneous injury that leads to worsening airway edema. This physiologic consequence can be deadly and may progress expeditiously.[6] It is thus paramount to obtain and sustain a definitive airway early in treatment.[1]
There have been limited trials on the appropriate respiratory modes in patients with inhalation injury. A mechanical ventilation strategy shown to improve morbidity and mortality from ARDS and ALI comes from the ARDSNET trial, which showed in a large randomized controlled trial that lung protective strategies of limited tidal volumes of 6–8mL/kg and plateau pressures of less than 30cm H2O improved outcomes.[40]
Conventional mechanical ventilation modes, such as control mode ventilation, assist-control mode, synchronized intermittent mandatory ventilation, pressure control mode and pressure support mode are limited in the patient with inhalation injury.[18][41] Thus, in order to support these patients and apply lung-protective ventilation strategies in patients with inhalation injury, non-conventional ventilator modes are often employed.[42]
Popular among these non-conventional ventilator modes are high-frequency percussive ventilation (HFPV), high frequency oscillatory ventilation (HFOV), airway pressure release ventilation (APRV), extracorporeal membrane oxygenation (ECMO). HFPV has however shown the most promising results.[1]
Physiotherapy[edit | edit source]
Studies have shown that a combination of techniques such as gravity-assisted bronchial drainage with chest percussion and vibrations. are effective in secretion removal.[43][44][45]
Bronchial Drainage/Positioning[edit | edit source]
Bronchial drainage/positioning is a therapeutic modality which uses gravity-assisted positioning designed to improve pulmonary hygiene in patients with inhalation injury or retained secretions. Due to skin grafts, donor sites, and the use of air fluid beds, clinical judgment might influence the most appropriate decisions.[6] In fact, positioning in the Trendelenburg and various other positions may acutely worsen hypoxemia. Evidence has shown that a patient’s arterial oxygenation may fall during positioning.[45]
Percussion[edit | edit source]
It aids the removal of secretions from the tracheobronchial tree. There should be a padding between the patient and the percussor’s hand in order to prevent irritation of the skin.[6] Percussion is applied over the surface landmarks of the bronchial segments which are being drained. Incisions, skin grafts, and bony prominences should be avoided during percussion.[46]
Vibration/shaking[edit | edit source]
Vibration/shaking a shaking movement used to move loosened secretions to larger airways so that they can be coughed up or removed by suctioning. Vibration involves rapid shaking of the chest wall during exhalation. Mechanical vibrations have been reported to produce good clinical results. Gentle mechanical vibration may be indicated for patients who cannot tolerate manual percussion.[6]
Early Ambulation[edit | edit source]
Early ambulation is another effective means of preventing respiratory complications. With appropriate use of analgesics, even patients on continuous ventilatory support can be taken out of bed and placed into a chair. The sitting position has several beneficial effects which include:[6]
- The patient can breathe with regions of the lungs which are normally hyperventilated
- Muscular strength and tone are preserved
- Contractions are prevented and exercise tolerance is maintained
Prognosis[edit | edit source]
While mortality rates for inhalation injury has not changed significantly over the last fifty years, the improvements in standards of care for severe burn injuries have.[1] Supportive strategies are vital in the management of inhalation injury, yet, large multi-centered trials are needed to demonstrate consistent results for many of the pharmacological adjuncts. HFPV as an unconventional mode of ventilation shows the most promising results and address the physiologic derangements from inhalation injury.[1]
Conclusion
Inhalation injury requires a robust knowledge of its pathophysiology to guide accurate diagnosis and drive the right therapeutic strategies. Practitioners must carefully work within available evidence for best outcomes from inhalation injuries –a classic determinant of mortality in severe burn.
References[edit | edit source]
- ↑ 1.0 1.1 1.2 1.3 1.4 1.5 1.6 1.7 1.8 1.9 Jones SW, Williams FN, Cairns BA, Cartotto R. Inhalation injury: pathophysiology, diagnosis, and treatment. Clinics in plastic surgery. 2017 Jul 1;44(3):505-11.
- ↑ 2.0 2.1 2.2 2.3 Dries and EndorfScandinavian Journal of Trauma, Resuscitation and Emergency Medicine2013,21:31
- ↑ 3.0 3.1 Kadri SS, Miller AC, Hohmann S, Bonne S, Nielsen C, Wells C, Gruver C, Quraishi SA, Sun J, Cai R, Morris PE. Risk factors for in-hospital mortality in smoke inhalation-associated acute lung injury: data from 68 United States hospitals. Chest. 2016 Dec 1;150(6):1260-8.
- ↑ Reper P, Heijmans W. High-frequency percussive ventilation and initial biomarker levels of lung injury in patients with minor burns after smoke inhalation injury. Burns. 2015; 41:65–70. [PubMed: 24986596]
- ↑ Al Ashry HS, Mansour G, Kalil AC, Walters RW, Vivekanandan R. Incidence of ventilator associated pneumonia in burn patients with inhalation injury treated with high frequency percussive ventilation versus volume control ventilation: A systematic review. Burns. 2016 Sep 1;42(6):1193-200.
- ↑ 6.00 6.01 6.02 6.03 6.04 6.05 6.06 6.07 6.08 6.09 6.10 Mlcak RP, Suman OE, Herndon DN. Respiratory management of inhalation injury. burns. 2007 Feb 1;33(1):2-13.
- ↑ Pruitt BA, McManus AT. The changing epidemiology of infection in burn patients. World journal of surgery. 1992 Jan 1;16(1):57-67.
- ↑ Tan A, Smailes S, Friebel T, Magdum A, Frew Q, El-Muttardi N, Dziewulski P. Smoke inhalation increases intensive care requirements and morbidity in paediatric burns. Burns. 2016 Aug 1;42(5):1111-5.
- ↑ Burn Incidence Fact Sheet. American Burn Association; 2016 National Burn Repository. American Burn Association; 2017.
- ↑ Foncerrada G, Culnan DM, Capek KD, González-Trejo S, Cambiaso-Daniel J, Woodson LC, Herndon DN, Finnerty CC, Lee JO. Inhalation injury in the burned patient. Annals of plastic surgery. 2018 Mar;80(3 Suppl 2):S98.
- ↑ 11.0 11.1 11.2 11.3 McCall JE, Cahill TJ. Respiratory care of the burn patient. Journal of Burn Care & Rehabilitation. 2005 May 1;26(3):200-6.
- ↑ Pruitt Jr BA, Flemma RJ, DiVincenti FC, Foley FD, Mason Jr AD, Young Jr WG. Pulmonary complications in burn patients: a comparative study of 697 patients. The Journal of thoracic and cardiovascular surgery. 1970 Jan 1;59(1):7-20.
- ↑ Palmieri TL. Inhalation injury: research progress and needs. Journal of burn care & research. 2007 Jul 1;28(4):549-54.
- ↑ Trunkey DD:Inhalation injury. Surg Clin North Am1978,58:1133–1140
- ↑ Moore SJ, Ho K, Hume AS. Severe hypoxia produced by concomitant intoxication with sublethal doses of carbon monoxide and cyanide. Toxicology and applied pharmacology. 1991 Jul 1;109(3):412-20.
- ↑ 16.0 16.1 16.2 16.3 Traber, D., Herndon, David, Enkhbaatar, Perenlei, Maybauer, Marc, Maybauer, Dirk. The pathophysiology of inhalation injury. In: Herndon, D., editor. Total Burn Care. Fourth. Saunders Elsevier; 2012. p. 219-28
- ↑ 17.0 17.1 17.2 17.3 17.4 17.5 17.6 17.7 Sousse LE, Herndon DN, Andersen CR, Ali A, Benjamin NC, Granchi T, Suman OE, Mlcak RP. High tidal volume decreases adult respiratory distress syndrome, atelectasis, and ventilator days compared with low tidal volume in pediatric burned patients with inhalation injury. Journal of the American College of Surgeons. 2015 Apr 1;220(4):570-8.
- ↑ 18.0 18.1 18.2 Jones, SW., Ortiz-Pujols, Shiara M., Cairns, Bruce. Smoke inhalation injury: a review of the pathophysiology, management, and challenges of burn-associated inhalation injury. In: Gilchrist ICaEYC. , editor. Current Concepts in Adult Critical Care Society of Critical Care Medicine. Vol. 2011. 2011.
- ↑ Friedl HP, Till GO, Trentz O, Ward PA. Roles of histamine, complement and xanthine oxidase in thermal injury of skin. The American journal of pathology. 1989 Jul;135(1):203.
- ↑ Granger DN. Role of xanthine oxidase and granulocytes in ischemia-reperfusion injury. American Journal of Physiology-Heart and Circulatory Physiology. 1988 Dec 1;255(6):H1269-75.
- ↑ Granger DN, Kvietys PR. Reperfusion injury and reactive oxygen species: the evolution of a concept. Redox biology. 2015 Dec 1;6:524-51.
- ↑ Baile EM, Dahlby RW, Wiggs BR, Pare PD. Role of tracheal and bronchial circulation in respiratory heat exchange. Journal of Applied Physiology. 1985 Jan 1;58(1):217-22.
- ↑ Moritz AR, Henriques Jr FC, McLean R. The effects of inhaled heat on the air passages and lungs: an experimental investigation. The American journal of pathology. 1945 Mar;21(2):311.
- ↑ Herndon DN, Barrow RE, Traber DL, Rutan TC, Rutan RL, Abston S. Extravascular lung water changes following smoke inhalation and massive burn injury. Surgery. 1987 Aug 1;102(2):341-9.
- ↑ Walker PF, Buehner MF, Wood LA, Boyer NL, Driscoll IR, Lundy JB, Cancio LC, Chung KK. Diagnosis and management of inhalation injury: an updated review. Critical Care. 2015 Dec;19(1):1-2.
- ↑ 26.0 26.1 You K, Yang HT, Kym D, Yoon J, Cho YS, Hur J, Chun W, Kim JH. Inhalation injury in burn patients: establishing the link between diagnosis and prognosis. Burns. 2014 Dec 1;40(8):1470-5.
- ↑ Pitt BR, Radford EP, Gurtner GH, Traystman RJ. Interaction of carbon monoxide and cyanide on cerebral circulation and metabolism. Archives of Environmental Health: An International Journal. 1979 Sep 1;34(5):354-9.
- ↑ Moylan JA, Chan CK. Inhalation injury--an increasing problem. Annals of surgery. 1978 Jul;188(1):34.
- ↑ Putman CE, Loke JA, Matthay RA, Ravin CE. Radiographic manifestations of acute smoke inhalation. American Journal of Roentgenology. 1977 Nov 1;129(5):865-70.
- ↑ Wanner A, Cutchavaree A. Early recognition of upper airway obstruction following smoke inhalation. American Review of Respiratory Disease. 1973 Dec;108(6):1421-3.
- ↑ Moylan JA, Adib K, Birnbaum M. Fiberoptic bronchoscopy following thermal injury. Plastic and Reconstructive Surgery. 1975 Nov 1;56(5):598.
- ↑ Moylan Jr JA, Wilmore DW, Mouton DE, Pruitt Jr BA. Early diagnosis of inhalation injury using 133 xenon lung scan. Annals of surgery. 1972 Oct;176(4):477.
- ↑ Lange M, Hamahata A, Traber DL, Cox RA, Kulp GA, Nakano Y, Traber LD, Herndon DN, Enkhbaatar P. Preclinical evaluation of epinephrine nebulization to reduce airway hyperemia and improve oxygenation after smoke inhalation injury. Critical care medicine. 2011 Apr;39(4).
- ↑ Dries DJ, Endorf FW. Inhalation injury: epidemiology, pathology, treatment strategies. Scandinavian journal of trauma, resuscitation and emergency medicine. 2013 Dec 1;21(1):31.
- ↑ Jonkam C, Zhu Y, Jacob S, Rehberg S, Kraft E, Hamahata A, Nakano Y, Traber LD, Herndon DN, Traber DL, Hawkins HK. Muscarinic receptor antagonist therapy improves acute pulmonary dysfunction after smoke inhalation injury in sheep. Critical care medicine. 2010 Dec 1;38(12):2339-44.
- ↑ van der Poll T, Coyle SM, Barbosa K, Braxton CC, Lowry SF. Epinephrine inhibits tumor necrosis factor-alpha and potentiates interleukin 10 production during human endotoxemia. The Journal of clinical investigation. 1996 Feb 1;97(3):713-9.
- ↑ Suter PM, Domenighetti G, Schaller MD, Laverrière MC, Ritz R, Perret C. N-acetylcysteine enhances recovery from acute lung injury in man: a randomized, double-blind, placebo-controlled clinical study. Chest. 1994 Jan 1;105(1):190-4.
- ↑ Villegas L, Stidham T, Nozik-Grayck E. Oxidative stress and therapeutic development in lung diseases. Journal of pulmonary & respiratory medicine. 2014 Aug;4(4).
- ↑ Acute Respiratory Distress Syndrome Network. Ventilation with lower tidal volumes as compared with traditional tidal volumes for acute lung injury and the acute respiratory distress syndrome. New England Journal of Medicine. 2000 May 4;342(18):1301-8.
- ↑ Petrucci N, Iacovelli W. Lung protective ventilation strategy for the acute respiratory distress syndrome. Cochrane Database of Systematic Reviews. 2007(3).
- ↑ Fitzpatrick JC, Cioffi Jr WG. Ventilatory support following burns and smoke-inhalation injury. Respiratory care clinics of North America. 1997 Mar 1;3(1):21-49.
- ↑ Chopra SK, Taplin GV, Simmons DH, Robinson Jr GD, Elam D, Coulson A. Effects of hydration and physical therapy on tracheal transport velocity. American Review of Respiratory Disease. 1977 Jun;115(6):1009-14.
- ↑ Marini JJ, Pierson DJ, Hudson LD. Acute lobar atelectasis: a prospective comparison of fiberoptic bronchoscopy and respiratory therapy. American Review of Respiratory Disease. 1979 Jun;119(6):971-8.
- ↑ Oldenburg Jr FA, Dolovich MB, Montgomery JM, Newhouse MT. Effects of postural drainage, exercise, and cough on mucus clearance in chronic bronchitis. American Review of Respiratory Disease. 1979 Oct;120(4):739-45.
- ↑ 45.0 45.1 Remolina C, Khan AU, Santiago TV, Edelman NH. Positional Hypoxemia in Unilateral Lung Disease. Survey of Anesthesiology. 1982 Feb 1;26(1):1.
- ↑ Herndon DN, Inhalation injury. In: Total burn care; 2nd ed., 2002, p. 242–53.