Heterotopic Ossification
Original Editors - Bruce Tan as part of the from Bellarmine University's Pathophysiology of Complex Patient Problems project.
Top Contributors - Bruce Tan, Morgan Yoder, Hannah McCabe, Naomi O'Reilly, Lucinda hampton, Admin, Elaine Lonnemann, 127.0.0.1, Wendy Walker, WikiSysop and Kim JacksonDefinition/Description[edit | edit source]
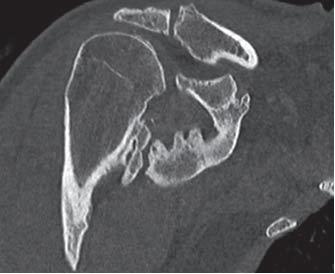
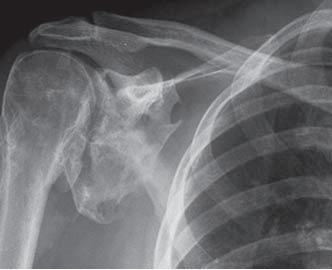
Heterotopic Ossification (HO) refers to the formation of lamellar bone inside soft tissue structures where bone should not exist. The development of HO is extra-articular and occurs outside the joint capsule. The new bone generally does not involve the periosteum. The HO may also attach to the cortex of adjacent bone, with possible cortical disruption.[1] This process can occur in structures such as the skin, subcutaneous tissue, skeletal muscle, and fibrous tissue adjacent to bone. In more rare forms, HO has been described in the walls of blood vessels and intra-abdominal sites such as the mesentery.[2] The primary risk factor for HO is inciting trauma.[1] Additionally, inflammation has been shown to play a role in the formation of HO due to osteoprogenitor cells being stimulated to proliferate in that environment.[3] Additional factors are described in further detail in the Etiology section. Although HO can be found at any site, it is most prevalent in the major joints such as the hip, elbow, shoulder and knee.
HO was first described by Patin in 1692 while working with children diagnosed with myositis ossificans progressiva.[4] In 1918, Dejerine & Ceillier detailed the anatomical, clinical, and histological features of ectopic bone formation in soldiers who sustained spinal injuries during World War I.[5]
Following the work of Dejerine & Ceillier, Marshall Urist described the osteoinducive properties of bone morphogenic protein in ectopic areas such as muscle. This was, and still is, considered a "landmark discovery" in orthopedic research.[6] Two of the original research articles by Urist can be found below:
Etiology/Causes
[edit | edit source]
The exact pathophysiology of HO is unknown. The transformation of primitive mesenchymal cells in connective tissue into osteoblastic tissue and osteoid involve diverse and poorly understood triggers.[1] These triggers include genetic, post-traumatic, neurogenic, post-surgical, and reactive lesions of hands and feet.
Genetic forms include two types: Fibrodysplasia Ossificans Progressiva (FOP) and Progressive Osseous Heteroplasia(POH). These types are described as massive deposits of heterotopic bone around multiple joints in the absence of an inciting event (i.e. trauma). This is the most severe type of HO, progressively forming throughout the life and severely effecting health, life expectancy, and quality of life.[2]
Post-traumatic HO begins with spindle cell proliferation within the first week of the traumatic event. Within 1-2 weeks, primitive osteoid develops. After the second week, primitive cartilage and woven bone can be seen. Trabecular bone forms 2-5 weeks after the trauma. Amorphous calcium phosphate is gradually replaced by hydroxyapatite crystals as the mineralization progresses. After about 6 months, there is an appearance of true bone in the connective tissue between the muscle planes.[1]
Neurogenic heterotopic ossification occurs after sickle cell anemia, hemophilia, tetanus, poliomyelitis, multiple sclerosis, and toxic epidermal necrolysis. Neurogenic HO develops only in sites distal to the level of the spinal cord injury. The areas affected by HO are almost always on the affected side of brain injury or stroke.[1]
Post-surgical HO most commonly develops after procedures which require open reduction, internal fixation and joint replacement surgeries, with THA being the most common.[2]
Reactive lesions of the hands and feet are usually associated with the periosteum or periarticular fibrous tissue, which differentiates the category from myositis ossificans. These lesions occur in three clinico-radiologic settings: bizzare parosteal osteochondroma
tous, florid reactive periositis, and subungual exostoses.[2]
Prevalence[edit | edit source]
• Following lower extremity amputation: 7%[6]
• Following TBI: 11% (ranges reported from 10-20%)[6]
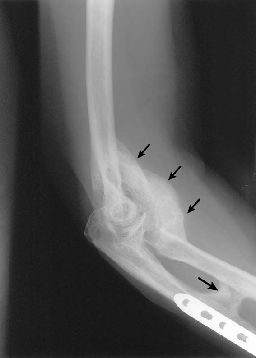
Hip most common, followed by elbow[1][7]
• Following SCI: 20% (ranges reported from 20-40%)[6]
• Following THA: 55%[1]
• Following elbow fracture and/or dislocation: 90%[8]
Following forearm fracture: 20%
In a recent study, Foruria et al. looked at the prevalence of heterotopic ossification in all elbow pathologies entering an emergency room over a 5 year period. They found the highest prevalence of HO occurred in terrible triad injuries, transolecranon fracture-dislocations, and an associated distal humeral fracture. The location of the ossification was most commonly found on the posterior aspect of the ulna. Risk factors for HO include dislocation or subluxation at the time of injury, open injury, and severe chest injury. When a pathology was treated surgically, 37% of participants developed an ossification.[7]
Clinical Diagnosis
[edit | edit source]
Clinical signs and symptoms of HO may appear as soon as 3 weeks or up to 12 weeks after initial musculoskeletal trauma, spinal cord injury, or other precipitating events.[9] The first sign of HO is generally loss of joint mobility and subsequently loss of function. Other findings that may suggest the presence of HO include swelling, erythema, heat, local pain, palpable mass, and contracture formation. In some cases, a fever may be present.[1]
Differential Diagnoses: The initial inflammatory phase of HO may mimic other pathologies such as cellulitis, thrombophlebitis, osteomyelitis, or a tumorous process.[3][4] Other differential diagnoses include DVT, septic arthritis, hematoma, or fracture. DVT and HO have been positively associated. This is thought to be due to the mass effect and local inflammation of the HO, encouraging thrombus formation. The thrombus formation is caused by venous compression and phlebitis.[1]
Classification Systems
[edit | edit source]
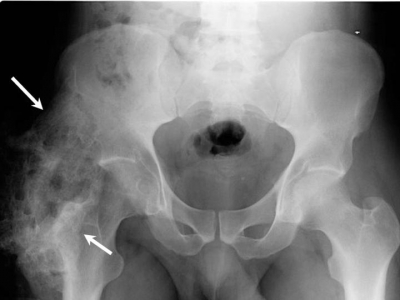
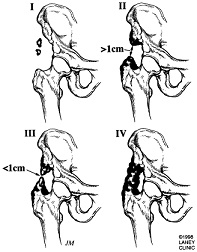
Brooker Classification of Heterotopic Ossification (Following THA)[10]
Class 1: Island of bone within a soft tissue about the hip
Class 2: Bone spurs originating from the pelvis of proximal end of femur leaving at least 1 cm between opposing bone surfaces
Class 3: Bone spurs originating from pelvis or proximal femur leaving <1 cm between opposing bone surfaces
Class 4: Ankyloses of the hip
Brooker did not describe a class 0 but subsequent studies using the Brooker classification have defined Class 0 as the absence of radiographic HO.
Schmidt and Hackenbrock Classification of Heterotopic Ossification (Following THA)[1]
Region 1: Heterotopic ossification strictly below the tip of the greater trochanter
Region 2: Heterotopic ossification below and above the tip of the greater trochanter
Region 3: Heterotopic ossification strictly above the tip of the greater trochanter
Grade A: Single or multiple heterotopic ossification <10 mm in maximal extent without contact with pelvis or femur
Grade B: Heterotopic ossification >10 mm without contact with the pelvis but with possible contact with the femur; no bridging from the femur to the proximal part of the greater trochanter; no evidence of ankyloses
Grade C: Ankylosis by means of firm bridging from femur to pelvis
McAfee’s Classification of Heterotopic Ossification (Following Total Disc Arthroplasty)[1]
0: No HO
1: Islands of bone not within the margins of the disc and not interfering with motion
2: Bone within the margins of the disc but not blocking motion
3: Bone within the margins of the disc and interfering with motion of the prosthesis
4: Bone ankylosis
Stages of Development
[edit | edit source]
Chronology of Development of Heterotopic Ossification[2]
- 0 days: +/- erythema, swelling, tenderness
- 7 days: clinically palpable mass
- 7-14 days: poorly defined shadow on radiograph
- 14-21 days: osteoid deposition, radiographic shadows
- 21-35 days: fluffy radiodensities; the “dotted veil” effect
- 24 days: definite radiographic evidence
- 30 days: mineralization shows a zonal pattern (best seen on CT scan)
- 45 days: histologic “zonal” pattern evident, reflecting well-formed mineralization at the periphery
- 180-365 days: development of mature bone
Diagnostic Tests and Lab Tests[edit | edit source]
Ultrasonography[3]
• Detection of HO 2 weeks earlier than by x-ray
• More accurate than any laboratory tests
• Help clinicians advocate for prompt/aggressive physical therapy
• Eliminates high false-positive rate of physical exam alone
Three-phase bone scintigraphy[4]
• Diagnostic and therapeutic follow-up purposes
• Phases 1 and 2 are indicative of hyperemia and blood pooling (precursors to process of HO)
• Usually positive after 2-4 weeks
• Serial bone scans used to monitor metabolic activity of HO to determine optimal timing for surgical resection and to predict postoperative occurrence
Radiography[1]
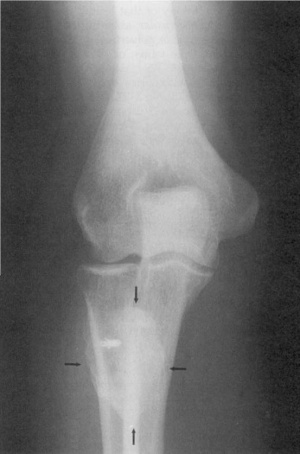
• Soft tissue mass is the earliest radiographic finding
• HO seen on radiographs 4-6 weeks post-injury has a typical appearance of circumferential ossification with a lucent center
• Cannot detect the mineralization of HO during first weeks after trauma/onset
• Frequently used to classify HO following THA
• Differential diagnosis: avulsion fracture fragments, osteochondral bodies, nonosseous soft tissue calcification and osteosarcoma
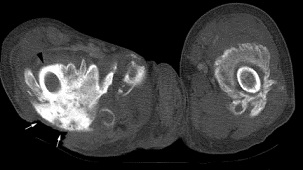
MRI and CT Scan[1]
• MRI not routinely used for evaluation of HO
• CT may detect soft tissue ossification at earlier stages than standard radiograph
Three-phase technetium-99m (99mTc) methylene diphosphonate bone scan[1]
• Most sensitive imaging modality for early detection and assessing the maturity of HO
• Can use to monitor the metabolic activity of HO and determine appropriate time for surgery and predict postoperative recurrence
• Usually positive >2 weeks before radiographic evidence of HO
Prostaglandin E2: (PGE2)[4]
• Monitior PGE2 excretion in 24-hour urinalysis
• PGE2 felt to be reliable bone marker for early detection and determining treatment efficacy
• A sudden increase is an indication for bone scintigraphy
Alkaline Phosphatase: (ALP)[5]
• Frequently used in early detection of HO
ALP values are increased in early HO and plateau at ~4 weeks[3]
• Cannot be used to draw clinical conclusions about maturity or recurrence of HO
• Acute fractures often have similar ALP values, limiting the usefulness of ALP values in diagnosis of HO[3]
Elevated Creatine Kinase Levels[1]
• Correlate with histologic involvement of muscle and severity of disease
• Not specific to HO
• May predict a higher risk for HO development and severity
• May be helpful in planning and evaluation of response to treatment
Matrix Metalloproteinase-9[3]
• Marine animal models suggest this could be an early biomarker for HO formation
Clinical Note: the lack of simple objective measures in detecting heterotopic bone formation causes HO to be misdiagnosed in the early stages, leading to delayed treatment.[6]
Systemic Involvement[edit | edit source]
Evidence shows that there are no systemic effects secondary to the formation of heterotopic ossification. The most common sites where this condition presents are the hips, knees, spine, elbow, wrists, hands, and any site which is involved in a traumatic event.
Associated Co-Morbidities
[edit | edit source]
The most common conditions found in conjunction with heterotopic ossification:[4] [2]
- Ankylosing spondylitis
- Rhuematoid arthritis[7]
- Hypertrophic osteoarthritis
- Diffuse idiopathic skeletal hyperostosis
- Paget's disease
- Quadriplegia and paraplegia
Medical Management (Current Best Evidence)[edit | edit source]
The treatment of heterotopic ossification is largely dependent on the amount of ectopic bone formation, the location and the associated functional limitations of the patient.
The first goal of medical management is to identify those patients at risk for developing HO and treating them prophylactically. Research supports two other approaches for the medical management of HO: 1)surgical excision and 2) radiation therapy.
Prophylactic Treatment:
Further research needs to be done in this area, however there are currently experimental options. These include:
Local radiation therapy
- Decreased incidence of all Brooker classes of HO following THA, but has greater effect on preventing Brooker classification 3 and 4 compared to NSAIDS
- Post-operative better than pre-operative[3]
- The use of radiotherapy as a prophylactic treatment comes mainly from the literature concerning total hip arthroplasty.[6]
- Radiating pluripotential mesenchymal cells may effectively prevent the development of HO. [5]
- A dose of 700-800 cGy of local radiation in the first four post-operative days has shown to prevent HO formation in patients who are at high risk.[6]
Oral indomenthacin
Medications:
Medications are prescribed to patients who are at risk for developing heterotopic ossification for preventative measures and to aid in the treatment after formation of heterotopic lesions. The two types of medications shown to have both prophylactic and treatment benefits are as follows:
Non-steroidal anti-inflammatory drugs: NSAIDS
Indomethacin (two-fold action)
1. Inhibition of the differentiation of mesenchymal cells into osteogenic cells (direct)
2. Inhibition of post-traumatic bone remodeling by suppression of prostaglandin-mediated response (indirect) and anti-inflammatory properties
Biphosphonates:
Three-fold action
1. Inhibition of calcium phosphate precipitation
2. Slowing of hydroxyapatite crystal aggregation
3. Inhibition of the transformation of calcium phosphate to hydroxyapatite
Clinical Note: Clinicians must be aware of potential complications (mainly GI related) with patients taking NSAIDS on a routine basis.
Sugical intervention:
The two main goals of surgical intervention are to alter the position of the affected joint or improve its range of motion (ROM).[5] Through his work, Garland has created a recommended timetable for surgical intervention:[4]
- 6 months following traumatic development of HO
- 1-year following development of HO secondary to a spinal cord injury
- 18 months following development of HO secondary to head injury
The above timetables were established to determine the most optimal timing of surgical intervention. Clinicians must determine if the lesion has reached maturation before surgical excision to decrease the risk of intraoperative complications such as hemorrhage, and the reoccurrence of the ectopic lesion.[9] The use of bone scans to determine metabolic activity of the lesion and serum ALP levels are common aids in this decision making process.
Shehab et al. describes criteria for recommending surgical removal of heterotopic ossification. The criteria are as follows:[9]
- Significantly limited ROM of involved joint (e.g., hip should have < 50 deg ROM) for most patients, progression to joint ankylosis is the most serious complication of heterotopic ossification.
- Absence of local fever, swelling, erythema, or other clinical findings of acute heterotopic ossification.
- Normal serum alkaline phosphate levels.
- Return of bone scan findings to normal or near normal; if serial quantitative bone scans are obtained, there should be a sharply decreasing trend followed by a steady state for 2-3 months.
Rehabilitation Post-Operatively
It is recommended that a rehabilitation program should start within the first 24 hours after surgery. The program should last for 3 weeks to prevent adhesion.[7]
Physical Therapy Management
[edit | edit source]
Physical therapy has been shown to benefit patients suffering from heterotopic ossification. Pre-operative PT can be used to help preseve the structures around the lesion. ROM exercises (PROM, AAROM, AROM) and strengthening will help prevent muscle atrophy and preserve joint motion.
Clinical note: caution must be taken when working with patients with known heterotopic lesions. Therapy which is too aggressive can aggravate the condition and lead to inflammation, erythema, hemorrhage, and increased pain.
Post-operative rehabilitation has also shown to benefit patients with recent surgical resection of heterotopic ossification. The post-op management of HO is similar to pre-op treatment but much more emphasis is placed on edema control, scar management, and infection prevention. Calandruccio et al. outlined a rehabilitation protocol for patients who underwent surgical excision of heterotopic ossification of the elbow. The phases of rehab and goals for each phase are as follows:[6]
Phase I (Week 1)
Goals:
- Prevent infection
- Protect and decrease stress on surgical site
- Decrease pain
- Control and decrease edema
- ROM to 80% of affected joint
- Maintain ROM of joint proximal and distal to surgical site
Phase II (2-8 weeks)
Goals:
- Reduce pain
- Manage edema
- Encourage limited ADL performances
- Promote scar mobility and proper remodeling
- Promote full ROM of affected joint
- Encourage quality muscle contractions
Phase III (9-24 weeks)
Goals:
- Self-manage pain
- Prevent flare-up with functional activities
- Improve strength
- Improve ROM (if still limited)
- Return to previous levels of activity
Casavant and Hastings also provide great insight into the evaluation and management of heterotopic ossification in their article titled Heterotopic Ossification about the Elbow: A Therapist’s Guide to Evaluation and Management.[7]
Clinical Note: The two studies used above were primarily focused on rehabiliatation of the elbow secondary to heterotopic ossification. However, the goals and stages of the rehabilitation process can be used as a guide when treating at other sites.
Case Report
[edit | edit source]
Title: Heterotopic Ossification Causing Radiculopathy after Lumbar Total Disc Arthroplasty
Key Words: Heterotopic Ossification, physical therapy, total disc arthroplasty, radiculopathy
Word Count: 520
Authors: Keith L. Jackson, Justin M. Hire, Jeremy M. Jacobs, Charles C. Key and John G. DeVine (modified by Morgan Blake and Hannah McCabe)
Abstract:
This case details the complications one man experienced due to the development of post-operative Heterotopic Ossification in his lumbar spine.
Introduction:
A total disc arthroplasty is a relatively new treatment strategy for lumbar discogenic back pain that has shown promising short- and intermediate-term results. Specific complications that can accompany total disc arthroplasty include vertebral body fracture, heterotopic ossification (HO), implant malposition, and early or late component extrusion. This case details a posterior component placement contributed to heterotopic bone growth within the spinal canal, causing neural impingement and radiculopathy and ultimately requiring component extraction, decompression, and lumbar arthrodesis.
Case Presentation:
- Subjective: 45 y/o male presents to the clinic with new onset R leg pain. He denies paresthesias or the loss of motor function, and he also denied a prior history of HO formation, trauma, or inflammatory arthritis. His PMH includes L5/S1 lumbar total disc arthroplasty 2 years ago for discogenic back pain. He reports that following surgery he had a significant reduction in his symptoms but developed this new pain about 6 months ago.
- Demographic Information: 45 years old, Male, works for UPS as a mail carrier
- Medical diagnosis: suspicion of HO
- Co-morbidities: HTN, DM
- Previous care/treatment: physical therapy (minimal improvements were noted)
- Self-report outcomes: Oswestry: 46% (Severe Disability)
- Objective: Physical examination revealed limited lumbar ROM in forward flexion due to R leg pain, positive single leg raise test, strength was 5/5 for all major muscle groups, intact sensation, and normal (2+) reflexes.
Patient was referred to his primary care provider who performed additional testing. Results of additional testing were:
- Radiographs demonstrated implant encroachment into the spinal canal with heterotopic bone formation outside the margins of the disc.
- Computed Tomography Myelogram revealed compression of the traversing nerve root secondary to the inferior endplate of the implant that resided posterior to the margin of the vertebral endplate as well as an associated posterior bone growth further into the canal.
Clinical Impression:
Patient demonstrates decreased lumbar ROM, with a history of trauma to the lumbar spine due to the lumbar disc arthroplasty. These factors and the positive imaging resulted in the patient being diagnosed with HO.
Intervention:
Patient elected to undergo surgical removal of his lumbar arthroplasty with fusion of L5/S1 and HO decompression. Patient returned to physical therapy post operatively for recovery.
Outcomes:
6 weeks post operatively patient reports complete relief of his radicular leg pain. Radiographs demonstrate a solid fusion without recurrence of HO formation.
Discussion:
As a physical therapist it is important to consider HO in your differential diagnosis when treating patients with a history of trauma or surgery. It is especially important to remember the signs and symptoms of HO when treating patients with limited ROM. We may be the first health professional to recognize the development of HO.
Acknowledgements:
Case adapted from “Heterotopic Ossification Causing Radiculopathy after Lumbar Total Disc Arthroplasty”[8]
Case Studies[edit | edit source]
Tibiofibular Syndesmosis and Ossification. Case Report: Sequelae of Ankle Sprains in an Adolescent Football Player.
Heterotopic Mesenteric Ossification ("intraabdominal myositis ossificans): A Case Report.
A Case of Psoas Ossification from the use of BMP-2 for Posterolateral Fusion at L4-L5.
- Brower RS, Vickroy NM. A case of psoas ossification from the use of BMP-2
for posterolateral fusion at L4–L5.Spine 2008; 18: 653-655.<span id="fck_dom_range_temp_1299789628126_629" />
Infrapatellar Heterotopic Ossification after Anterior Cruciate Ligament Reconstruction.
- Valencia H, Gavín C. Infrapatellar heterotopic ossification after anterior cruciate ligament reconstruction. Knee Surgery, Sports Traumatology, Arthroscopy: Official Journal Of The ESSKA [serial on the Internet. (2007, Jan), [cited March 31, 2011]; 15(1): 39-42. Available from: MEDLINE.]
Heterotopic Ossification of the Ulnar Collateral Ligament: A Description of a Case in a Top Level Weightlifting Athlete.
- A Giombini, L Innocenzi, G Massazza, F Fagnani, et al. Heterotopic ossification of the ulnar collateral ligament: a description of a case in a top level weightlifting athlete. Journal of Sports Medicine and Physical Fitness. 2005 Sep 1;45(3): 370-80. In: ProQuest Medical Library [database on the Internet [cited 2011 Mar 31]. Available from: ProQuest; Document ID: 942792311]
Resources
[edit | edit source]
The Mystery of Heterotopic Ossification and How it Affected My Life
References[edit | edit source]
- ↑ 1.00 1.01 1.02 1.03 1.04 1.05 1.06 1.07 1.08 1.09 1.10 1.11 1.12 1.13 1.14 Mavrogenis AF, Soucacos PN, Papagelopoulos PJ. Heterotopic Ossification Revisited. Orthopedics. 2011Jan;34(3):177.
- ↑ 2.0 2.1 2.2 2.3 2.4 2.5 McCarthy EF, Sundaram M. Heterotopic ossification: a review. Skeletal Radiol 2005; 34: 609-619.
- ↑ 3.0 3.1 3.2 3.3 3.4 3.5 3.6 Firoozabadi R, Alton T, Sagi HC. Heterotopic Ossification in Acetabular Fracture Surgery. Journal of the American Academy of Orthopaedic Surgeons. 2017;25(2):117–24.
- ↑ 4.0 4.1 4.2 4.3 4.4 4.5 Bossche LV, Vanderstraeten G. Heterotopic ossification: a review. J Rehabil Med 2005; 37: 129-136.5. Pape HC et al. Current concepts in the development of hetetrotopic ossification. Journ Bone and Joint Surg 2004; 86: 783-787.
- ↑ 5.0 5.1 5.2 5.3 Pape HC et al. Current concepts in the development of hetetrotopic ossification. Journ Bone and Joint Surg 2004; 86: 783-787.
- ↑ 6.0 6.1 6.2 6.3 6.4 6.5 6.6 6.7 Hsu JE, Keenan MA. Current review of heterotopic ossification. UPOJ 2010; 20: 126-130.
- ↑ 7.0 7.1 7.2 7.3 7.4 Foruria AM, Augustin S, Morrey BF, Sanchez-Sotelo Joaquin. Heterotopic Ossification After Surgery for Fractures and Fractures-Dislocations Involving the Proximal Aspect of the Radius or Ulna. The Journal of Bone and Joint Surgery, Incorporated. 2015May15;95-A(10):e66(1)-e66(7).
- ↑ 8.0 8.1 Dalury DF, Jiranek WA. The incidence of heterotopic ossification after total knee arthroplasty. Journal of Arthroplasty 2004; 19: 447-457.
- ↑ 9.0 9.1 9.2 Shehab D, Elgazzar AH, Collier BD. Heterotopic ossification. Jour of Nuclear Medicine 2002; 43: 346-353.
- ↑ Hug KT, Alton TB, Gee AO. In Brief: Classifications in Brief: Brooker Classification of Heterotopic Ossification After Total Hip Arthroplasty. Clinical Orthopaedics and Related Research®. 2014;473(6):2154–7.