Epigenetics
Original Editor - Lucinda hampton
Top Contributors - Lucinda hampton and Chelsea Mclene
Introduction[edit | edit source]
Epigenetics is the study of heritable and stable changes in gene expression that occur through adjustments in the chromosome rather than in the DNA sequence. They do not directly alter the DNA sequence, rather epigenetic mechanisms are able to regulate gene expression through chemical modifications of DNA bases and changes to the chromosomal superstructure.[1]
Epigenetics affect which genes are expressed, and subsequently whether the cells should produce relevant proteins, e.g. determining a cell’s specialisation such as skin cell, blood cell, hair cell, liver cells, etc. These effects play a role both as a foetus develops through gene expression (active) or repression (dormant), and also through nurture with environmental stimuli having the ability to cause genes to be turned off or turned on.[2] The different combinations of genes that are turned on or off is what makes each one of us unique. For example, if we have brown or black hair, how sociable you are, how an oyster tastes to us. Additionally, there are indications that some epigenetic changes can be inherited.[2]
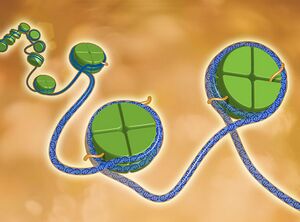
Image 1: Epigenetics describes modifications to the genome that can be passed on to future cells.These changes do not alter the nucleotide sequence of the DNA-the As, Gs, Ts, and Cs that make up our genes. Rather, they modify the "backbone" that supports the DNA sequence. These modifications influence when and how often a gene is active.
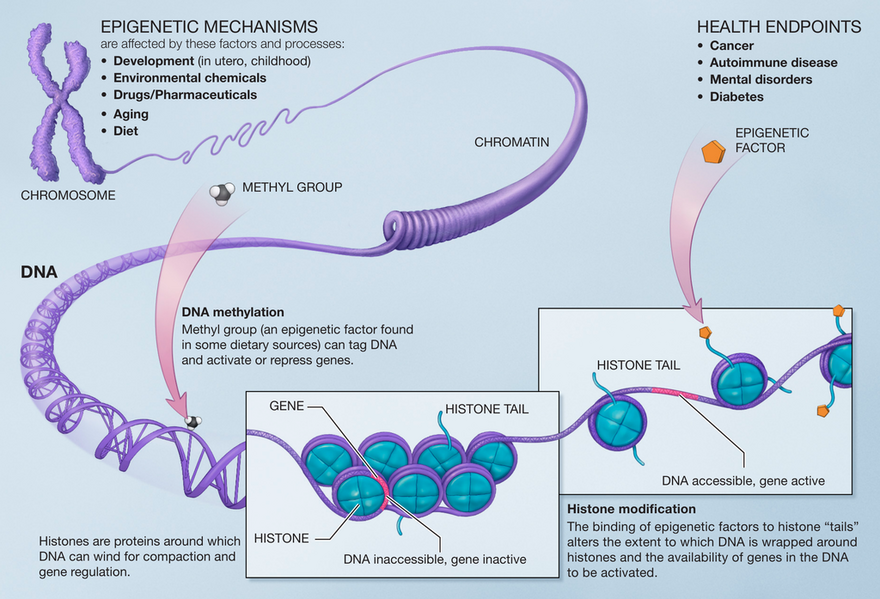
Epigenetics Mechanisms[edit | edit source]
Epigenetic changes effects work in different ways to bring about gene expression. Types of epigenetic changes include:
- DNA Methylation: refers to a chemical reaction in the body in which a small molecule called a methyl group gets added to DNA, proteins, or other molecules. The addition of methyl groups can affect how some molecules act in the body. In DNA the group is usually added to specific places, blocking the proteins that attach to DNA to “read” the gene. When this chemical group is removed the process called is called demethylation. In most cases, methylation turns genes “off” and demethylation turns genes “on.”
- Histone modification: DNA wraps around histones (which are proteins). If the histones are wrapped tightly around the DNA they cannot be accessed by proteins that “read” the gene. The genes that are wrapped around histones are turned “off” and the genes that are not wrapped around histones and are turned “on.” Chemical groups can be added or removed from histones and change whether a gene is unwrapped ("on") or wrapped (“off”).
- Non-coding RNA: DNA directs for making coding and non-coding RNA. Coding RNA is used to make proteins. Non-coding RNA helps control gene expression which it does attaching to coding RNA, along with certain proteins, to break down the coding RNA so that it cannot be used to make proteins. Non-coding RNA may also recruit proteins to modify histones to turn genes “on” or “off.”[3]
Epigenetics began over 60 years ago. During the 1970s it started becoming more prominent with the arrival of molecular biology and during the last 10–15 years, has emerged as a stand-alone discipline complementary to genetics. [4]
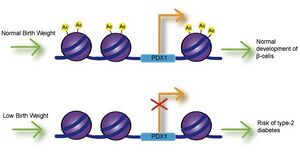
Image 4: PDX1 is an essential transcription factor for proper development and function of pancreatic beta cells. Poor maternal nutrition is linked to intrauterine growth restriction (IUGR) and low birth weight, and can result in decreased expression of PDX1 through decreased histone acetylation at the PDX1 proximal promoter. Reduced expression of PDX1 may result in improper formation of beta cells and increase the risk of type-2 diabetes in the offspring
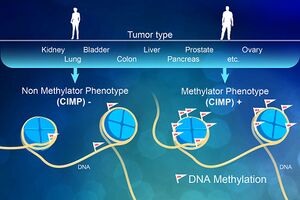
Image 5: In every cell, DNA is tagged with chemical groups-like flags on the DNA-that make up the epigenetic code and act as guideposts. These flags help the cell's machinery know when to activate or inactivate a gene. In healthy cells, these flags are correctly placed and genes are expressed in the right proportions. However, in many tumor cells the flags are incorrectly applied. One form of incorrect DNA flagging occurs when extreme amounts of otherwise healthy methyl chemical groups, which act like on/off switches for a gene, are incorrectly added to many places on the cell's DNA. Instead of a cell chugging along with its natural, biological jobs, these newly added methyl groups cause the cell to act erratically, leading to the cell expressing genes at the wrong levels, or not at all. This generally disrupts the normal flow of information in the cell. Importantly, these flags can turn off key defensive genes that help protect the cell from becoming cancerous and disrupt other processes. When this occurs, it's called "CpG Island Methylation Phenotype," or CIMP for short.
For a brief explanation watch this 5 minute video.
Epigenetics and Exercise Effects[edit | edit source]
It is now established that exercise is an important “medicine”. Examples of the role exercise can play in epigenetics include:
- Lifelong physical activity is associated with promoter hypo-methylation of genes involved in metabolism, myogenesis, contractile properties and oxidative stress resistance in aged human skeletal muscle[6]. Promoter regions are regions of DNA where transcription of a gene is initiated[7].
- Exercise is able to attenuate or reverse some of the high‐fat diet associated methylation patterns. Many of the genes found to be differentially methylated are implicated in metabolic functions, such as regulation of oxidative metabolism and glucose transportation, which have obvious significance for offspring.
- Exercise has also been shown to impact cognitive development, as hippocampal DNA methylation was found to be lower in exercise‐offspring. This shows that epigenetic intergenerational outcomes appear to be consistent with epigenetic alterations in people who regularly exercise[8].
- Studies show that acute and chronic exercise interventions induce a number of epigenetic modifications within the person exercising. In human skeletal muscle, both whole genome methylation and methylation of promoter regions of key metabolic genes decreased after completing a single‐session of cycling at 80% of VO2peak. Similarly, three months of regular single‐knee extension exercise showed genome‐wide DNA methylation alterations that were not observed in the untrained leg[8].
- Studies show that an important modulation of exercise exists on the epigenetics mechanisms, particularly in DNA methylation, specially of regular physical exercise[9].
Lifestyle Factors[edit | edit source]
In utero epigenetic changes are very sensitive as the epigenetic profile of the foetus as it is forming and developing. Teratogens like cigarette smoke, alcohol, and specific minerals having the ability to induce utero epigenetic changes.
As people age, the most significant influence on the epigenome is the environment. These include diet, smoking, physical activity and psychological stress[1].
The field of nutriepigenomics looks into how food and epigenetics work together to influence health and wellbeing. eg studies have found that certain compounds within the foods we consume could protect again cancer by adjusting methyl marks on oncogenes or tumor suppressor genes[2].
References[edit | edit source]
- ↑ 1.0 1.1 Al Aboud NM, Tupper C, Jialal I. Genetics, epigenetic mechanism. Available:https://www.ncbi.nlm.nih.gov/books/NBK532999/ (accessed 25.7.2022)
- ↑ 2.0 2.1 2.2 What is epigenetics A Super Brief and Basic Explanation of Epigenetics for Total Beginners Available: https://www.whatisepigenetics.com/what-is-epigenetics/(accessed 25.7.2022)
- ↑ CDC Epigenetics Available:https://www.cdc.gov/genomics/disease/epigenetics.htm (accessed 26.7.2022)
- ↑ W.W. Weber, in Comprehensive Medicinal Chemistry II, 2007 Available:https://www.sciencedirect.com/referencework/9780080450445/comprehensive-medicinal-chemistry-ii (accessed 26.7.2022)
- ↑ Carlos Guerrero-Bosagna. What is epigenetics. Available from: https://m.youtube.com/watch?v=_aAhcNjmvhc[last accessed 28/7/2022]
- ↑ Sailani, M.R., Halling, J.F., Møller, H.D., Lee, H., Plomgaard, P., Pilegaard, H., Snyder, M.P. and Regenberg, B., 2019. Lifelong physical activity is associated with promoter hypomethylation of genes involved in metabolism, myogenesis, contractile properties and oxidative stress resistance in aged human skeletal muscle. Scientific reports, 9(1), pp.1-11.
- ↑ Addgene Promoters Available:https://www.addgene.org/mol-bio-reference/promoters/ (accessed 26.7.2022)
- ↑ 8.0 8.1 Axsom JE, Libonati JR. Impact of parental exercise on epigenetic modifications inherited by offspring: A systematic review. Physiological Reports. 2019 Nov;7(22):e14287. Available:https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6874781/ (accessed 26.7.2022)
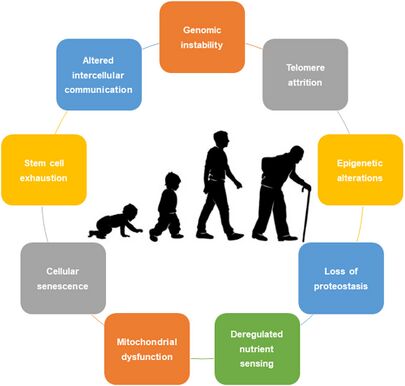
- ↑ Rebelo-Marques A, De Sousa Lages A, Andrade R, Ribeiro CF, Mota-Pinto A, Carrilho F, Espregueira-Mendes J. Aging hallmarks: the benefits of physical exercise. Frontiers in endocrinology. 2018 May 25;9:258.