Pain Descending Pathways
- Please do not edit unless you are involved in this project, but please come back in the near future to check out new information!!
- If you would like to get involved in this project and earn accreditation for your contributions, please get in touch!
Original Editor - Adrian Mallows.
Top Contributors - Adrian Mallows, Lucinda hampton, Jo Etherton, Admin and Lauren Lopez
What is the descending pain modulatory system?[edit | edit source]
The "top down" modulation of pain has been in evidence since the early work of Sherrington[1] showing that nociceptive reflexes were enhanced after transection of the spinal cord. This was further elaborated on by Fields[2] and Milan[3] who, based upon observations in the 1960's that electrical stimuation of the periaqueductal gray (PAG) area can produce analgesia, demostrated through electrophysiological and pharmacological studies that descending influences on spinal nociceptive processing involves the PAG and the rostral ventromedial medulla (RVM).
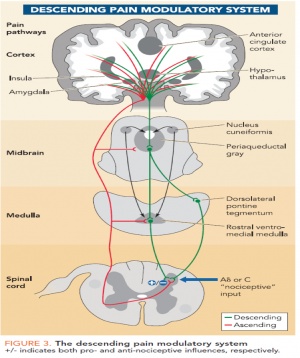
Work by Hadjipavlou et al[4] used functional and anatomical studies to link the descending pain modulatory system from the brain stem (where the PAG and RVM reside) to a number of higher level brain areas including; cingulofrontal regions, the amygdalae and the hypothalamus (figure 3[5]). This may go someway to help explain the role that emotions and cognition have in processing nociceptive information.
Underpinning the descending pain modulatory system is the endogenous opoid system[6] and according to Willer[7] this system may be activated by a variety of reflex and cognitively trigged states. At the spinal cord (dorsal horn) level, the opiod system causes inhibition of substance P from peripheral noxious mechanical stimulation[8] via release of noradrenaline from the dorsalateral PAG (dPAG) and thermal nociceptive stimuli via the release of serotonin from the ventrolateral PAG (vPAG)[9].
Why is the system useful?[edit | edit source]
Evidence for pain modulalatory mechanisms were first recorded by Beecher[10]. Beecher, a physician serving the US Army during World War II, observed as many as three quarters of badly wounded soldiers reported none to only moderate pain and did not require pain relief medication. According to his report the men were alert and responsive and the injuries were not trivial, including compound fractures and penetrating wounds. This led him to the conclusion that "strong emotions" block pain. This clearly opposess the classical Cartesian view where pain was considered to be a hard-wired system that passively transmitted noxious inputs to the brain. It is now generally accepted that the experience of pain does not soley rely on noxious inputs, but many variables interplay with the experience, including memory, mood, environment, attention and expectation. Ultimatley, this means the resultant pain experienced to the same sensory input can vary considerably[11]. It is the brain's job to weigh all the information and decide whether creating pain is the most appropraite reponse. This provides a neccesary survival function since it allows the pain experience to be altered according to the situation rather than having pain always dominate[11].
How can this help physiotherapists?[edit | edit source]
Knowledge of the descending pain modulatory system and its components can help physiotherapists in several ways. Firstly, it helps physiotherapists explain why the amount of pain a patient is experiencing does not neccesarily relate to the amount of tissue damage they have sustained[10]. Physiotherapists can educate their patients about the role of the descending pain modulatory system and how the central nervous sytem weighs all the information before deciding if a pain experience is the most approriate action for survival. Neuroscience education has been shown to be effective in several studies[12][13][14][15] [16][17].
Secondly, knowledge of the anatomy (see "what is the descending pain modulatory system" section) involved in the descending pain modulatory system can help physiotherapists utilise management strategies to that access and activate the system. These could include adding distractions to exercises and perfoming exercises in different emotional states and or in different environments.
Thirdly, manual techniques such as joint mobilisations, manipulations have been proposed to activate the system and significantly contribute to their therapeutic effects[18]. Noxious stimuli can activate the system[19][20] and this can help explain why manual techniques that may elicit some pain (to some degree) can be helpful to reduce pain overall. This knowledge can aid the physiotherapist with careful selection and use of techniques with a "top down" philosphy, freeing them from selecting interventions based merely on proposed local tissue reponses such as inhibiting reflex muscle contraction, reducing intra-articular pressure and reducing the level of joint afferent activity[21]
References[edit | edit source]
References will automatically be added here, see adding references tutorial.
- ↑ Sherrington CS. The Integrative Action of the Nervous System. New Haven, CT: Yale Univ. Press,1906.
- ↑ Fields HL. Pain modulation: expectation, opoid analgesia and virtual pain. Prog Brain Res 2000; 122:245-253;
- ↑ Milan MJ. Descending control of pain. Prog Neurobiology 2002; 66:355-474;
- ↑ Hadjipavlou G, Dunckley P, Behrens TE, Tracey I. Determining anatomical connectives between cortical and brainstem pain processing regions in humans: a diffusion tensor imaging study in healthy controls. Pain 2006; 123: 169-178
- ↑ Bingel U, Tracey I. Imaging CNS Modulation of Pain in Humans. Physiology 2008; 23:371-380
- ↑ Akil H, Watson SJ, Young E, Lewis ME, Khachaturian H, Walker JM. Endogenous opioids: biology and function. Annu Rev Neurosci 1984; 7: 223-255
- ↑ Willer JC, Dehen H, Cambier J. Stress induced analgesia in humans: endogenous opiods and naloxone-reversible depression of pain reflexes. Science 1981; 212:689-691
- ↑ Kuraishi, Y. Neuropeptide-mediated transmission of nociceptive information and its regulation. Novel mechanisms of analgesics 2008; 110(10),711-772
- ↑ Kuraishi Y, Harada Y, Aratani S. Seperate involvement of the spinal noradrenergic and serotonergic systems in morphine analgesia: the differences in mechanical and thermal algesic tests. Brain Res 1983;273, 245-252
- ↑ 10.0 10.1 Beecher HK. Pain in men wounded in battle. Ann Surg. 1946;123(1):96-105
- ↑ 11.0 11.1 Bingel U, Tracey I. Imaging CNS modulation of pain in humans. Physiolohy 2008;23:371-380
- ↑ Moseley G Combined physiotherapy and education is efficacious for chronic low back pain. Australian Journal of Physiotherapy 2002; 48:297–302
- ↑ Louw A, Louw Q, Crous LCC. Preoperative Education for Lumbar Surgery for Radiculopathy. South African Journal of Physiotherapy. 2009;65(2):3-8.
- ↑ Moseley, GL. A randonmised controlled trial of intensive neurophysiology education in chronic low back pain. Clin J Pain 2002;20:324-330
- ↑ Meeus MJ. Pain physiology education improves pain beliefs in patients with chronic fatigue syndrome compared with pacing and self-management education:a double-blind randomised controlled trial. Arch Phys Med Rehabil 2010;91:1153-1159
- ↑ Clarke CL. Pain Neurophysiology education for the management for the management of individuals with chronic low back pain: systematic review and meta-analysis. Manual Therapy 2011;16:544-549
- ↑ Louw A. The effect of neuroscience education on pain and disability, anxiety, and stress in chronic musculoskeletal pain. Archives of Physical Medicine and Rehabilitation 2011;92:2041-2056
- ↑ Wright A. Hypoalgesia post manipulative therapy: a review of a potential neurophysiological mechanism. Man Ther 1995;1(1), 11-16
- ↑ Yaksh TL, Elde RP. Factors governing release of methionine enkephalin-like immunoreactivity from mesencephalon and spinal cord of the cat in vivo. J.Neurophysiol 1981;46 (5), 1056-1075
- ↑ Fields HL, Basbaum AL. Central Nervous System mechanisms of pain modulation, In: Wall PD, Melzack R. Textbook of pain, 4th ed.Churchill Livingstone, Edinburgh; 1999
- ↑ Zusman M. Spinal manipulative therapy: a review of some proposed mechanisms, and a new hypothesis. Aust J. Physiotherapy 1986;32(2),89-99