Corticospinal Tract
Original Editor - Kate Sampson
Top Contributors - Kate Sampson, Matt Ross, Lucinda hampton, Kim Jackson, Anas Mohamed, WikiSysop, Joao Costa, Evan Thomas, Vidya Acharya, Carina Therese Magtibay, 127.0.0.1 and Wendy Walker
Description[edit | edit source]
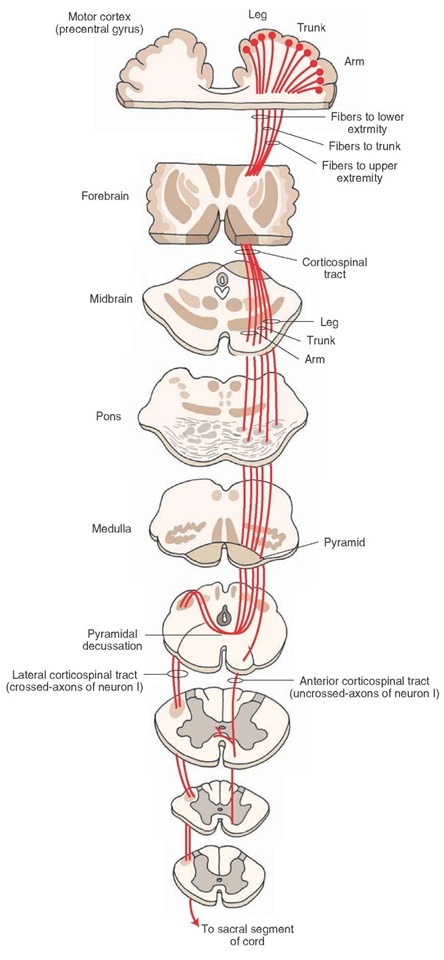
The corticospinal tract (CST) forms part of the descending spinal tract system that originate from the cortex or brainstem (Crossman & Neary, 2015[1]) and is also known as the pyramidal tract.
The CST has approximately 1 million nerve fibres with an average conduction velocity of approximately 60m/s using glutamate as their transmitter substance.
The CST descends through the corona radiata and posterior limb of the internal capsule to reach the brainstem. Here, it gives fibres that activate the motor cranial nerve nuclei, also known as the corticobulbar tract, that is responsible for the muscles of the face, jaw and tongue
The descending corticospinal tract descends from the origin:
- Through the corona radiata
- Posterior half of lateral ventrical (lower limb represented by posterior fibres, face most anterior)
- Posterior limb of internal capsule (lower limb represented by posterior fibres, face most anterior)
- Enters midbrain through cerebral peduncle (face represented by medial fibres, foot lateral and hand in the middle
- Enter medulla where they form medullary pyramids on either side of midline:
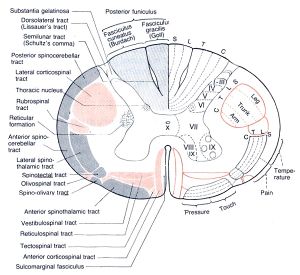
- Lateral fibres (lateral corticospinal tract) are contralateral fibres. These make up between 75-90% fibres. They descend in the posterior part of the lateral funiculus. This tract is detected through to the lumbosacral spine and fibres synapse either directly on anterior horn cells of the contralateral side to their origin (ipsilateral to their side of descent in the spinal cord), or on interneurones of layers within this same side.
- Anterior fibres (Anterior corticospinal tract) makes up between 10-25% of fibres. They descend ipsilaterally, however decussate near to their termination. Therefore these fibres continue to innteravate the contralateral side of the spinal cord. [2][3]
Of all corticospinal fibres approximately 20% terminate at thoracic levels, 25% at lumbosacral levels and 55% at cervical levels. Many of the fibres that originate from the motor cortex then terminate in the ventral horn of the spinal cord. [2]
Function[edit | edit source]
The CST has many functions which include the control of afferent inputs, spinal reflexes and motor neuron activity, the most important being the mediation of voluntary distal movements (Welniarz et al, 2016[4]). Outputs from the primary motor cortex (M1) contribute to the CST, making connections to excitatory monosynaptic alpha motor neurons, polysynaptic connections onto gamma motor neurons which are responsible for the control of muscle spindle length and polysynaptic connections via interneurons within the spinal cord (Shumway-Cook and Woollcott, 2007[5])
Recent developments have increased the understanding of the origin and termination of the CST neurons:
- 30%-40% arise from the primary motor cortex.
- Rest of the fibers arise from the supplementary motor area (SMA), premotor cortex (PMA), parts of the somatosensory areas (S1 and S2) and parts of the posterior parietal cortex.
Due to the various origins that contribute to the CST, it is considered that this tract not only forms part of the motor system, but also has a large sensory role also. These fibers that originate from the sensory cortex terminate in the dorsal horn of the spinal cord where they synapse with interneurons that receive input from somatosensory receptors and are thought to regulate information from peripheral receptors within the spinal cord. Therefore, the CST may act as a ‘gate’, modulating or inhibiting information that is deemed useful or irrelevant (Bassoe Gjelsvik & Syre, 2016[6]).
Clinical relevance[edit | edit source]
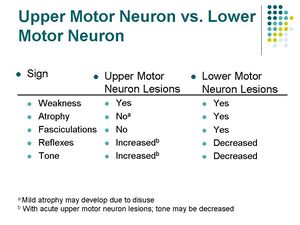
A lesion to the CST can occur anywhere along its path from the cortex to the anterior horn of the spinal cord. A lesion of the CST typically results in the upper motor neuron signs.
A lesion of the CST cranial to the decussation of the pyramids will result in deficits on the contralateral side.
A lesion of the CST caudal to the decussation of the pyramids will reslt in deficits on the ipsilateral side.
Stroke / TBI[edit | edit source]
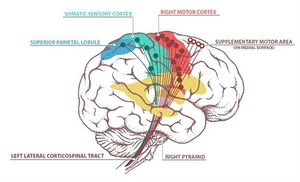
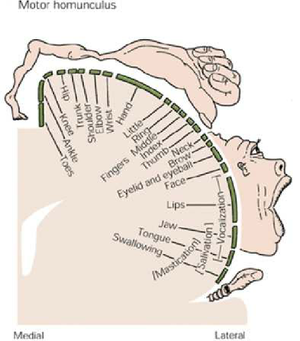
The image below depicts the motor homunculus. Dependant on what aspect of this is damaged will result in motor deficits on the contralateral side of the body.
Click here to read more about stroke.
Spinal Cord[edit | edit source]
Following a spinal cord injury, both voluntary (sensory and motor) and involuntary control can be impaired and the extent of recovery dependent on the severity of the lesion (Freund et al, 2013)[7]. As the CST has already decussated, motor deficits will be ipsilateral to the site of the lesion.
The ASIA outcome measure, which assesses both motor and sensation, will provide an indication of the level of the spinal cord lesion and whether or not it is complete or incomplete.
Crozier et al (1991) concluded that 89% of those who were ASIA B-E with pinprick preservation went on to ambulate. This is due close proximity of the spinothalamic tract to the lateral corticospinal tract and their shared blood supply.
Learn more about spinal cord injury.
Assessment[edit | edit source]
The effect of a lesion to the CST causes more than just muscle weakness. It also affects synergistic movement patterns that affect things such as dexterity, ambulation and activities of daily living.
There are a number of outcome measures than can be used dependent on what you want to assess. These include:
- Fugl-Meyer Assessment of Motor Recovery after Stroke (FMA)
- Oxford Muscle Grading System
- Stroke Rehabilitation Assessment of Movement (STREAM)
- Action Research Arm Test (ARAT)
- Cherokee Arm and Hand Activity Inventory
- Functional Ambulation Category
- Motor Assessment Scale
- Rivermead Mobility Index
- Rivermead Motor Assessment
- ASIA (Spinal Cord)
Read more about outcome measures in stroke rehabilitation by Salter et al (2013)[8]
Stinear et al (2007) suggested that Corticospinal Tract integrity could be used to identify the likely extent of motor recovery and may enable appropriate selection of rehabilitation strategies for individuals recovering from stroke [9]. In a further study conducted by Stinear et al (2012) they trialled the use of the PREP(predicting motor recovery) algorithm to assess the likelihood of upper limb recovery. By utilising the SAFE score (sum of the shoulder abduction and finger extension) 72 hours after stroke, Transcranial magnetic stimulation, motor
evoked potentials in affected upper limb or the Asymmetry Index (measured with diffusion-weighted MRI) they were able to predict whether there could be a complete- no recovery. It was suggested from these finding that clinicians using the PREP algorithm may be able to predict the likely extent of upper limb recovery and may be able to therefore manage of patient expectations from an earlier period.[10]
Treatment[edit | edit source]
Following a lesion to part of the corticospinal tract, such as a stroke, their function is impaired resulting in contralateral motor deficits. Although people begin to experience motor recovery to some extent, complete recovery is rarely achieved.
Following damage to the corticospinal tract, there is a cascade of events that occur at both a cellular and network level resulting in motor map reorganisation. This phenomenon is known as neuroplasticity, and it can be enhanced by rehabilitative training such as motor control and learning which is achieved by repetitive practice. Other treatment techniques may include:
- Gait Re-Education
- Mirror Therapy
- Constraint Induced Movement Therapy (CIMT)
- Task Specific Training
It is believed that during these activities that axonal remodelling may not only happen in the lesioned cortiospinal tract, but also the corticorubral tract from the ipsilesional hemisphere as the rubrospinal or the reticulospinal tract. It is thought that these deep brain areas provided support for the CST.
Another prooposed mechanism is an increased production of trophic factors as well as an increased density of trophic receptors on the neural surface, producing an environment more suitable for neural remodelling [11]
Resources[edit | edit source]
References[edit | edit source]
- ↑ Crossman, A.R. and Neary, D. (2015). Neuroanatomy. An illustrated Colour Text. 5th Edition. Churchill Linvingstone.
- ↑ 2.0 2.1 Crossman AR, Neary D. Neuroanatomy: An Illustrated Colour Text. Third Edition. London: Elsevier, 2004
- ↑ Bear MF, Connors BW, Paradiso. Neuroscience: Exploring the Brain Neuroscience: Exploring the Brain, Michael A. Paradiso. Edition 2, illustrated. Lippincott Williams & Wilkins, 2001
- ↑ Welniarz, Q., Dusart, I. and Roze, E., 2017. The corticospinal tract: Evolution, development, and human disorders. Developmental neurobiology, 77(7), pp.810-829.
- ↑ Shumway-Cook, A. and Woollcott, M.H. (2007). Motor Control. Translating Research into Clinical Practice. 3rd Edition. Lippincott Williams & Wilkins. USA.
- ↑ Bassoe Gjelsvik, B.E. and Syre, L. (2016). The Bobath Concept in Adult Neurology. 2nd Edition. Thieme Publishers. Germany.
- ↑ Freund, P., Curt, A., Friston, K. and Thompson, A., 2013. Tracking changes following spinal cord injury: insights from neuroimaging. The Neuroscientist, 19(2), pp.116-128.
- ↑ EBRSR (2013). Outcome measures in Stroke Rehabilitation. Available at: http://www.ebrsr.com/sites/default/files/Chapter%2020_Outcome%20Measures.pdf [Accessed 20th September 2018]/
- ↑ Stinear CM, Barber PA, Smale PR, Coxon JP, Fleming MK, Byblow WD. Functional potential in chronic stroke patients depends on corticospinal tract integrity. Brain. 2007 Jan 1;130(1):170-80.
- ↑ Stinear CM, Barber PA, Petoe M, Anwar S, Byblow WD. The PREP algorithm predicts potential for upper limb recovery after stroke. Brain. 2012 Aug 1;135(8):2527-35.
- ↑ Okabe, N., Narita, K. and Miyamoto, O. (2017). Axonal remodeling in the corticospinal tract after stroke: how does rehabilitative training modulate it?. Neural regeneration research, 12(2), p.185.