Congenital and Acquired Neuromuscular and Genetic Disorders
Original Editor Kimberley Foy, Susan Hamilton, Alannah Henderson, Sean Lewis, James Millar, Christine Mitchell, Clem Nihill
Top EditorsClem Nihill, Sean Lewis, Shaimaa Eldib, Kim Jackson, Christine Mitchell, Kimberley Foy, James Millar, Alannah Henderson, Laura Ritchie, Mande Jooste, 127.0.0.1, Rucha Gadgil, Lucinda hampton, Tarina van der Stockt, Admin, Evelin Milev, WikiSysop, Evan Thomas, Mila Andreew and Scott Buxton
Introduction[edit | edit source]
According to the WHO, congenital anomalies or birth defects affect one in every 33 infants every year worldwide and result in approximately 3.2 million birth defect-related disabilities every year. The prevalence of major congenital anomalies is 23.9 per 1,000 births for 2003-2007. 80% were live births. 2.5% of live births with congenital anomaly died in the first week of life. 2.0% were stillbirths or fetal deaths from 20 weeks gestation. 17.6% of all cases were terminations of pregnancy following prenatal diagnosis (TOPFA) according to data from EUROCAT (European Surveillance of Congenital Anomalies)[1]. Giving birth to a child with such disorders can happen to any mother regardless of age, racial or cultural heritage, socioeconomic status, health or lifestyle. What is Congenital and Acquired?
Congenital Disorder[edit | edit source]
A congenital disorder is one which exists at birth and very often before birth. It also can include those conditions which develop within the first month of birth. Congenital disorders vary widely in causation and abnormalities and can be as a result of genetic or chromosomal abnormalities, infection, birth trauma or the environment the fetus was in whilst in the uterus.
Acquired Disorder[edit | edit source]
Acquired disorders, on the other hand, develop after birth and can develop over the course of one’s life.
1-Cerebral Palsy[edit | edit source]
Cerebral Palsy (CP): is a general term for chronic non-progressive neurological conditions that affect a child's ability to move and to maintain posture and balance [2] .Damage to certain areas of the brain (before or after birth) that control movement and coordination. Every child diagnosed with CP will have unique signs and symptoms. Each case is unique. In general, CP children will not able to control certain muscles in their body the way they are intended to be controlled. It is estimated that 1 in 400 babies in the UK have a type of CP.[3] Every case of cerebral palsy is unique to the individual this is due to the type and timing of the injury to the developing brain.
For further explanation of Cerebral palsy, please visit these pages:
- Cerebral_Palsy_Introduction.
- Classification of Cerebral Palsy.
- Cerebral Palsy Outcome Measures.
- Cerebral Palsy and Sport.
- Category of : Cerebral_Palsy has many pages that help in understanding the condition.
2-Dystrophy[edit | edit source]
Muscular Dystrophy (MD) is a group of inherited conditions that have a steady degenerative progression[4] which causes muscles to become weak over time[5]. The muscle weakness begins in the legs most often[6]. Some forms of this disease can affect the heart and lungs, which can create life-threatening complications[5]. It is caused by a mutation in the genes responsible for muscle structure, which interferes with the child’s ability to function[5]. As the disease progresses, the level of disability becomes worse. Both boys and girls can be affected by muscular dystrophy, however some affect boys predominantly, such as Duchenne’s muscular dystrophy (DMD).
There are many types of muscular dystrophy. Each is classified based on their presentation
| Type | Prevalence | Common Symptoms |
|---|---|---|
| Duchenne Muscular Dystrophy | 1 in 3,500 | • Difficulty walking, running or jumping • Difficulty standing up • Learn to speak later than usual • Unable to climb stairs without support • Can have behavioural or learning disabilities |
| Facioscapulohumeral Muscular Dystrophy | 1 in 7,500 | • Sleeping with eyes slightly open • Cannot squeeze eyes shut tightly • Cannot purse their lips |
| Myotonic Dystrophy | 1 in 8000 | • Muscle stiffness • Clouding of the lens in the eye • Excessive sleeping or tiredness • Swallowing difficulties • Behavioural and learning disabilities • Slow and irregular heartbeat |
| Becker Muscular Dystrophy | Varies; 1 in 18,000 – 1 in 31, 000 |
• Learn to walk later • Experience muscle cramps when exercising |
| Limb-Girdle Muscular Dystrophy | Estimated to be in a range of 1 in 14,500 – 1 in 123,000 | • Muscle weakness in hips, thighs and arms • Loss of muscle mass in these same areas • Back pain • Heart palpitations / irregular heartbeats |
| Oculopharyngeal Muscular Dystrophy | 1-9 in 100,000 | • Does not usually appear until age 50-60 • Dropped eyelids • Trouble swallowing • Gradual restriction of eye movement • Limb weakness, especially around shoulders and hips |
| Emery-Dreifuss Muscular Dystrophy | 1 in 100,000 | • Develop symptoms in childhood and adolescence • Muscle weakness • Trouble on stairs • Tendency to trip • Slow, irregular heartbeat |
Duchenne Muscular Dystrophy (DMD)[edit | edit source]
Duchenne’s muscular dystrophy (DMD) is the most common type, and therefore will be the focus of the rest of this section. Duchenne’s is almost exclusively seen in boys, however, girls are carriers of the condition. The gene which results in DMD has been found on the X chromosome that the boy receives from his mother[12]. If the mother is a carrier of this mutated gene, the son has a 50% chance of developing DMD[13]. Signs & Symptoms There are no abnormalities seen at birth and any symptoms usually begin when the child starts walking[14]. Children with DMD generally have delayed motor milestones when compared with other children, for instance they begin walking around 18 months[14]. These children also have difficulty sitting and standing independently[15]. The disease becomes even more apparently between 4 and 5 years of age when the child is tripping and stumbling often and has trouble keeping up with other children[14].
The weakness of the child’s calf muscles and the muscles back of the thigh early in the condition causes the child to stand with a more pronounced curve in their lower back[4]. They do this in order to maintain balance as their centre of gravity has shifted[4]. This way of standing gives the child maximal support at both the hip and the knee[4].
The child stands with their legs further apart to give themself a broader base of support[4]. This contributes to the child developing an altered walking pattern referred to as a waddling gait[14].
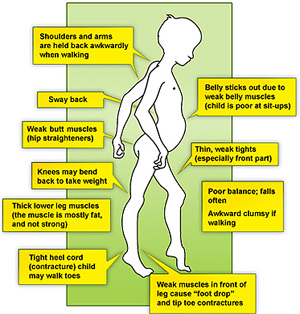
In this gait pattern, children walk on their toes with feet apart to help maintain balance, with an increased curve in the lower back[16].
Contractures are a classic finding in DMD[17]. It develops when tissues, such as muscle fibers, which are normally stretchy are replaced by hardened, non-stretchy tissue[17]. They are seen as a major cause of disability[18]. They prevent normal movement, and, for children with DMD, occur often in the legs, especially the calf and muscles around the hip[18].
Gower’s Sign is a very common physical finding for boys with Duchenne’s[19]. It involves using their hands to ‘climb’ up their legs in order to stand up[19]. It is due to a weakness in the child’s hip muscles[19].
Boys with DMD may also suffer from behavioural or mental deficits, but this is not always the case [20]. Any such impairments are not progressive meaning they will not get worse over time[21]. Delayed language milestones may be an early warning sign of DMD and the child may need to work with a speech and language therapist to overcome these difficulties[21].
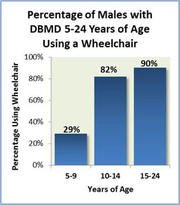
Between the ages of 6-11, there is a steady decline in muscle strength and by the age of 12 most children are wheelchair bound[22]. There are further complications surrounding children being dependent on wheelchairs, such as scoliosis and respiratory problems[23].
Medical Management[edit | edit source]
Sadly, there is no cure for Duchenne’s, but there are ways to help improve the individual’s quality of life and provide help for the stage they are in.
Mobility aids will be given to help the child be as independent as possible. This can include a walker in the beginning phases and can progress to a motorized wheelchair. In-home hoists are useful for the carers when they need help to transfer the child. Standing frames also become useful when the child can no longer stand on their own. This helps the child gain the benefits of standing, such as increased bone density, and stretching the muscles even if the child cannot stand on their own[24]. Knee-ankle-foot orthosis may be used as well. They have been found to prolong the child’s independent mobility[25]. These should be used alongside mobility aids, such as a zimmer frame [26]. Staying as active as possible is recommended. Bedrest can make the muscle-weakening worse[6].
Steroids are commonly prescribed for children with DMD, and are the only palliative treatment[27]. Steroids have been shown to increase the child’s muscle strength and their functional ability[28]. Steroids may help delay the child becoming wheelchair dependent, however, there may be side effects[28]. Another group of medications that has been shown to be helpful are Beta-Blockers[29]. Heart and respiratory function slowly decline in these children and these drugs are used to help manage both these problems[30].
Some families may consider surgery for their child. Common surgeries for Duchenne’s boys include foot surgery, insertion of a feeding tube and spinal surgeries to correct scoliosis, which may occur from being wheelchair dependent[31]. There are many facts to consider before surgery, such as the effect general anaesthesia has on the cardiac and respiratory systems, which are already compromised in children with DMD[32]. Families need to weigh the advantages of the surgery with the risk before making a decision. For example, evidence has been found that surgery to correct scoliosis has improved respiratory function[33], and it also improves the cosmetic appearance and comfort of the child [34], however depending on the child’s cardiac and respiratory function there may be concerns about how anaesthesia will affect them.
Physiotherapy Management[edit | edit source]
Physiotherapy is essential to the management of Duchenne’s. It is important to monitor the physical symptoms of the condition and physiotherapy can help keep the child active for as long as possible. Physiotherapists will work with the parents and carers and provide them with information and manual skills that will be helpful for the child.
Contractures are one of the major side effects that a physiotherapist will address. They will do these through a stretching routine, which can also be taught to the parents[35].
Physiotherapists will also be responsible for advising the parents on any orthoses, such as AFOs, and referring them with a paediatric orthotist[36]. They will also help families choose what mobility aids and equipment the child might need.
In the early stages of the condition, the physiotherapist will be involved in helping keep the child active. During later stages of the condition, the physiotherapist will help more with respiratory issues as well[36].
Physiotherapists will monitor the child’s posture in sitting, lying and standing[37]. They can inform the parents of ways to help the child sit, stand and lie in optimal positions using pillows or splints. A sleep system and night splints may be recommended for nighttime to help maintain the child’s posture over a long period of time[36].
Many physiotherapists use the NorthStar Amulatory Assessment in order to objectively monitor the child’s progression. Initiated in 2003, it is a tool designed specifically for children with DMD and has the child perform up to 17 activities, including standing, head-raising, hopping and running[38]. This assessment is used only for children who are still able to walk. It is standardized with each child given the same instructions and their ability given a score of 0-2[39]. It is easy to administer and can be completed in approximately 10 minutes[38]. These are useful when consulting with other medical practitioners and letting them know where the child is physically.
3-Charcot-Marie-Tooth disease[edit | edit source]
Charcot-Marie-Tooth disease (CMT) is known as a hereditary motor and sensory neuropathy (HMSN) and is the most common inherited neuromuscular disease with a prevalence of approximately 1 in every 2,500 [40]. CMT involves the degeneration of nerve fibres in the body that results in muscle weakness and wasting along with a decrease in sensation [41][42]. CMT is a slowly progressive disorder and it encompasses a large group of clinically and genetically heterogeneous disorders.
Causes[edit | edit source]
CMT is caused by genetic mutations with approximately 1000 mutations in 80 genes that are related to the physical presentation of the disease. The diagnosis and classification of CMT is a very complicated process[41].The two main types are Type I which is known as demyelinating CMT (CMT1) and Type II which is known as axonal CMT (CMT2)[40]. CMT1A is the most common form of CMT and it is caused by a duplication of the 1.4 Mb region of chromosome 17 containing the peripheral myelin protein 22(PMP22) gene[40]. More information regarding the specifics about the different gene mutations can be found here [43].
Signs & Symptoms[edit | edit source]
The signs and symptoms of CMT are extremely variable between each different type due the extensive amount of different mutations possible. Symptoms most often begin in adolescence or early adulthood, but can also begin later in mid-adulthood[44]. It is a progressive disease so the symptoms change between earlier and later stages.
Some of these signs & symptoms may include[45][44]:
- Fatigue (most common to all those affected): This is a direct result of having to put in additional effort with daily activities
- Early Signs:
- Difficulty walking or an awkward walking pattern: A child may have trouble lifting their feet which may result in tripping
- Clumsiness at a young age
- Lack of agility
- Common symptoms
- Pes- Cavus, also known as highly arched feet: This can cause foot and ankle instability issues which may result in ankle sprains.
- Very flat feet
- Curled or hammer toes: This can be very uncomfortable for a child and may cause pain and difficulty finding appropriate shoe.
- Lower legs are very thin, while the thigh muscles are a normal shape and bulk, or size:This characteristic is known as the inverted champagne bottle
- Some sensory loss and numbness in both the arms and legs :This is not usually a major problem for most individuals, but can result in unknown injuries if it is very severe
- Cold hands and feet due to poor circulation.
- Later symptoms
- Upper limbs including both the hands and forearms may be affected as the disease progresses
- Loss of fine motor control
- Loss of dexterity & overall hand strength
- Pain
- Tremors
CMT most often affects the distal limbs first, or those limbs further away from the body such as arms and legs. However, the lower limbs are usually affected before the upper limbs. The muscles in the lower part of the leg, ankle, and foot begin to atrophy as the disease progresses. This means the amount of muscle in these areas will decrease. Atrophy in the more proximal parts of the limbs or the parts closer to the body such as the thighs and upper arms is rare and usually only occurs in those more severely affected. As noted above, this muscle weakness along with a decrease in sensation can cause a lot of problems with both walking without any aid as well as problems with balance [46][47].
Medical Management[edit | edit source]
There is currently no cure or drug therapy for CMT. The main treatment options are rehabilitation therapy which will involve both a physiotherapist and an occupational therapist, and surgical treatment options[41].
Surgical Treatment[edit | edit source]
Surgical treatment is used for individuals with CMT with different skeletal deformities most often in their feet. Most individuals start out with flexible deformities in which the ankle begins to turn in, known as a cavovarus deformity. However, during the later stages of this disease, the deformity can become fixed. Treatment options are, therefore, soft tissue surgeries, osteotomies or removal of bone, and joint fusions. These can either be performed on their own or as a combination of a few. Research indicating who is appropriate for surgery and when this would be optimal is not yet conclusive as more in-depth long-term studies must be completed. For the upper limbs, tendon transfers may be beneficial to improve the ability to oppose the thumb and assist with wrist extension. In addition, scoliosis, also known as a curvature of the spine, is prevalent in about 15-20% of individuals with CMT and if severe enough, may need surgical intervention[41].
Physiotherapy Management[edit | edit source]
Physiotherapy is a key factor in a child’s management. It helps to improve the symptoms of CMT as well as decrease the risk of muscle contractures also known as the shortening of muscles. Physiotherapy will include low-impact exercises, posture and balance work, walking or swimming, and some strength training as well[48].
Physiotherapy along with occupational therapy should be started at the onset of symptoms. Starting physiotherapy early on can be beneficial because muscle weakness and sensory loss will be at a minimum[49].
The physiotherapist will also be involved in assessing the need for any additional aids or equipment. Many people with CMT may require high-top shoes or boots in order to provide more stability to the ankles and prevent injury. Others may need braces known as ankle-foot-orthoses (AFOs) which act similar to a cast, but they are removable. An AFO is a plastic brace, custom made for each individual to provide the necessary support and prevent tripping as a result of foot-drop. If individuals have more muscle weakness proximally or higher up the leg, then knee-ankle-foot orthoses are available.
The majority of individuals with CMT do not require to use of a wheelchair, however, in later stages of the disease some may choose to use one when going longer distances to reduce fatigue and the amount of stress on joints. The physiotherapist will work in conjunction with the occupational therapist and help with referrals to a podiatrist to ensure that all necessary equipment has been provided [46]. More information on different types of adaptive equipment can be found below.
Hydrotherapy[edit | edit source]
Hydrotherapy is a highly beneficial option as exercise for individuals with CMT. Essentially, it is just structured exercise lead by a physiotherapist that takes place in a pool. Hydrotherapy pools are not always easily accessible, but have many benefits especially for those individuals with CMT. Some of the benefits include reducing the amount of stress on joints due to the buoyancy of the water but still adds some light resistance to exercises in order to help maintain muscle strength[49]. More Information on hydrotherapy can be found below.
Aerobic Exercise[edit | edit source]
Exercise can also be carried out in a gym or at home. Walking, cycling, swimming and mild weight-training are all appropriate exercises that are safe to carry out with CMT. Overall, any type of general exercise will be beneficial for individuals with CMT. As long as that person is comfortable and working at low-moderate intensity, research shows that aerobic exercise can help to reduce fatigue, improve mood, and increase a person’s endurance. The goal is to help keep a child as independent as possible for as long as possible [49]. However, it is important to avoid overworking and exhaustion. When carrying out any type of exercising, fatigue and weakness should not be caused within 30 minutes of exercises and neither should any form of excessive muscle soreness or cramping. These are things that are strongly discouraged when exercising with CMT[50].
Stretching[edit | edit source]
Stretching is also a key part of physiotherapy. As noted above, individuals with CMT are at risk for muscle contractures and stretching assists in maintaining muscle length. When this is done slowly and gently it can provide comfort for individuals and reduce stiffness in the joints[50]. Physiologically. in a disease process such as CMT, where the progression of the condition is due to axonal and demyelinating factors in the peripheral nervous system, application of Proprioceptive Neuromuscular Facilitation (PNF) techniques with elastic or manual resistance, with low impact on the skeletal and muscular system could demonstrate improvement in the capacity of the muscle, without producing fatigue.
Balance and Posture[edit | edit source]
Balance is an extremely important ability in order to carry out daily tasks. It involves gathering sensory input from the body and its surroundings in order to accurately orientate itself with gravity and the environment. With CMT, sensation may be altered which will affect a person’s ability to balance and ultimately completely normal activities of daily living[51]. Tai Chi, Yoga, and Pilates are all very useful exercises for improving both balance and posture.
4-Spina Bifida[edit | edit source]
Spina Bifida occurs when there is a problem in the formation of the spine and spinal cord in the developing embryo. It is also known as “split spine” as this developmental fault creates a gap where the spinal column fails to close and protect the spinal cord and associated nerves. This can happen at any level of the spine but most commonly occurs in the lower back or lumbar region [52] The degree of spinal closure and the structures involved can vary in spina bifida and it can therefore be divided into 3 different types [53]:
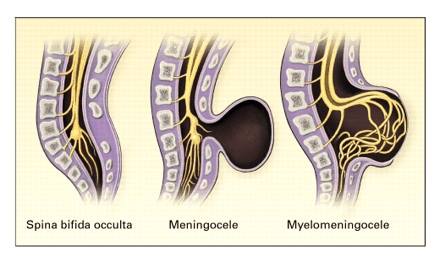
- Spina Bifida Occulta:
- Spina Bifida Occulta or Spina Bifida Aperta is the least severe form of spina bifida, where only the bony parts of the spine are affected. A small gap remains in the spine which is covered with skin. This can be identified after birth by a small area covered by a tuft of hair or an area of darker skin along the midline of the back. This form of spina bifida can go undetected as it is not associated with significant problems. It is also thought to be present in many healthy adults.
- Meningocoele:
- Meningocoele is the rarest form of spina bifida where the bones of the spine are affected along with the coverings of the spinal cord: the meninges. These can protrude through the gap in the spine to form a cyst, while the spinal cord and nerves remain in place within the spinal canal.
- Myelomeningocoele:
- Myelomeningocoele also know as Meningomyelocele or Spina Bifida Cystica is the most severe form of spina bifida, affecting 1 in 1000 births in the UK. Again, there is absence of closure of the vertebrae, however, this causes leaking of the meninges as well as the spinal cord outside the gap in the spinal column. As a result, a portion of the spinal cord, along with the spinal nerves, protrudes through the gap forming a cyst or sac that lies outside the body.
- Myelomeningocoele also know as Meningomyelocele or Spina Bifida Cystica is the most severe form of spina bifida, affecting 1 in 1000 births in the UK. Again, there is absence of closure of the vertebrae, however, this causes leaking of the meninges as well as the spinal cord outside the gap in the spinal column. As a result, a portion of the spinal cord, along with the spinal nerves, protrudes through the gap forming a cyst or sac that lies outside the body.
The exact cause of spina bifida is unknown but there may be associated genetic, environmental and dietary factors that can predispose develthe opment of the condition in certain individuals. The most commonly researched factor is the association of low levels of maternal folic acid. Folic acid is a vitamin present in many foods and is often added to breakfast cereals. It can also be found as a supplement in the pharmacy. It is thought to be involved in the development and formation of fetal cells and tissues, therefore, it is advised that folic acid should be taken daily in the upcoming months before conceiving and also during pregnancy [54].
Signs and Symptoms[edit | edit source]
Children with Spina Bifida can have a variety of symptoms which can vary from mild to severe. The main symptoms include:
- Cognitive symptoms - As spina bifida occurs due to problems with the developing spine and spinal cord, this can have an associated effect on development of the brain. Specifically areas of the brain involved in memory, learning, as well as concentration, understanding and the processing of language. Children may have difficulty with complex motor tasks such as tying laces where good visual and physical coordination is required [55].6 out of 10 children born with spina bifida will have normal intelligence levels, although around half of these will have some form of learning disability [56].
- Mobility Symptoms - The spinal cord allows information to travel up and down to brain in order to control movements made by muscles and joints. As the spinal cord and nerves can be compromised in certain types of Spina Bifida, there are often problems with muscle control and joint movement. In some cases, there may be paralysis of certain muscles which can result in the development of misshapen bones, particularly the feet, and abormal curving of the spine known as Scoliosis [57]. Those with severe mobility restrictions may also develop thin bones or osteoporosis due to the lack of use of the limbs [58]. The spinal cord and nerves also provide the brain with sensory information through touch. As the spinal nerves can be daged in some forms of Spina Bifida, there may be associated loss of sensation and feeling in the pelvic region and legs. This can cause problems with pressure sores and skin breakdown in infants who are unable to feel the need to change position [52]
- Incontinence (Urinary and Bowel) - The nerves travelling through the spinal cord also supply the bladder and bowel, ensuring the muscles within these organs can contract to contain urine and stools within the body. As a result, most children born with Spina Bifida will experience some degree of urinary and bowel incontinence [53]
Medical Management[edit | edit source]
Medical management of the newly born child with Spina Bifida varies according to the severity of their condition. Those with Spina Bifida Occulta do not ususally require any specific treatment. Some people with Spina Bifida Occulta do not exhibit any symptoms and may only discover they have the condition when they are older after having an XRAY. Children born with myelocoele or myelomeningocoele will require surgery normally within 2-3 days of birth in order to close the gap in the spine and return the spinal cord and nerves to their original place within the spinal column [52]. This aims to prevent infection and further damage to the exposed spinal cord and spinal nerves. Following surgery, the child will be monitored closely for signs of common pos-operative problems associated with this type of surgery, namely hydrocephalus and leaking of cerebrospinal fluid [53]
As the infant gets older, management of incontinence will be an important role of the medical team. Effective management strategies include the use of Clean Intermittent Catheterisation (CIC) and certain drugs which can increase the storage volume of the bladder [59]. Children can also develop constipation due to lack of bowel movements and will require development of a bowel programme which may involve assisted evacuation of stools. However, this will be based on an individualised assessment of the child and may involve educating the family in order to ensure the programme is effectively integrated into the child’s daily routine [60] Effective strategies in managing incontinence in children with spina bifida are extremely important in allowing them to socially integrate themselves as they get older and attend school [53].
Physiotherapy Management[edit | edit source]
The role of the physiotherapist in the early management of children with spina bifida is extremely important as it helps the child to develop an efficient and purposeful movement that can be incorporated into everyday tasks [61]. By optimising and maintaining mobility, this can eventually help children to become more independent as they get older. The physiotherapist will perform an initial assessment of the infants muscle strength and range of movement available at certain joints. This will allow the physiotherapist to determine which muscles are working properly and which ones are weak. This will give them a baseline measurement to use as a comparison as the child grows. This will also allow the physiotherapist to consider what problems the infant may have as they get older and what type of assistive devices or splints they may require when they begin to mobilise [53]. The physiotherapist will specifically be involved in:
- Joint Range of Motion
- In the early stages following surgery, the physiotherapist will begin passive range of motion exercises on the infant’s legs [52]. This will normally be performed 2-3 times a day. They will also demonstrate this technique to parents or carers so that they may continue to do these exercises as a home exercise programme when the infant is discharged. They may progress these exercises to mimic more functional movements which are related to normal everyday movement patterns. For example, whilst bending the left knee and hip, the right side will be kept straight as would happen in a normal walking pattern. These gentle exercises will help to maintain and may help increase the available range of motion available in joints where the movement restriction is mild. In those who have more pronounced restriction, the physiotherapist may advise that the number of exercise repetitions is increased and the movement is held for longer. The ultimate aim of range of motion exercises is to enable the child to learn and perform them independently as they grow up. It is important for the child to continue with these exercises because when they are moving independently, the functioning muscles may not be working through full range of motion. Passive range of motion exercises will therefore help to maintain flexibility and avoid the development of muscle tightenings known as contractures [52].
- Muscle strength.
- Altered muscle tone is a common symptom of spina bifida,therefore, physiotherapists use resistance training in order to strengthen these muscles that have been weakened. This is normally introduced when the infant is old enough to self mobilise. The physiotherapist can develop a programme of strength and endurance training which has been seen to improve functional abilities in children with spina bifida [62]. These training programmes may involve a variety of exercises for the upper and lower limbs, as well as muscles of the trunk and can help improve upper limb strength and cardiovascular fitness [63].
- Positioning and Handling
- Following the first few days after surgery, the infant will normally be placed inside or stomach lying. As the infant begins to stabilise and recover from surgery, the physiotherapist will offer advice as to how to hold the newborn child safely. This is incredibly important as the infant will have undergone major surgery which requires careful handling and positioning at all times. It may be advised that parents or carers hold the child underneath the stomach and across their forearm due to the surgical wound that will be present on the infant’s back. This handling technique may be used when sitting or walking around. When advised, parents or carers may take the infant for a walk around the hospital resting over the shoulder. This can encourage the child to begin to lift his or her head and begin to develop head and neck control [52].
- Mobility and Ambulation
- Mobility problems in children with spina bifida can vary according to the level of the spine that has been affected during development [64]. A child with a lesion in the lower back (Lumbar or Sacral levels), is more likely to be able to independently mobilise than one with a lesion in the upper thoracic spine. This can determine whether the child will require a wheelchair, orthotics or assistive devices [65].
- Parents and carers are often discouraged from using assistive devices such as infant walkers, jumpers and bouncer chairs as these can delay motor development. Infants require active movement and sensory information from the surrounding environment in order to learn how to move efficiently against gravity and maintain erect sitting and standing postures. This is no different for children with Spina Bifida. Infants with spina bifida benefit from movements that challenge control of the head, neck and torso, rather than the use of passive sitting devices or chairs. Active movement allows them to participate in the learning process. For example, rather than using a walker, parents are advised to physically hold their child in the standing position with as little support as possible to promote the necessary control of the legs and torso. This also allows the child to receive feedback from the floor and the surrounding environment [52].
- As the child begins to mobilise and ambulate more independently, he or she may be fitted for braces or splints to address any deformities caused by muscle imbalance or joint limitations. Orthoses such as braces and splints are supportive devices aimed at optimising existing muscle function and giving support where the child requires it. The earlier these are fitted and provided, the earlier the child will be prepared for the upright position required of standing and walking [66]. It therefore also enhances normal developmental progression and will eventually help the child take part in normal activities of their age group [52]. Children with Spina Bifida lesions in the upper thoracic regions of the spine may require bracing or splinting of the whole leg up to the level of the hip and chest. This is known as a Hip-Knee-Ankle-Foot Orthoses (HKAFO). Others may require orthotics aimed at stabilising the knee, ankle and foot. These are known as Knee-Ankle-Foot orthoses (KAFO) and Ankle-Foot Orthoses (AFO) [57] Reciprocal Gait Orthoses (RGO) may be also provided in order to promote a normal rhythmic walking pattern in the child [67] [66]. Children may require the additional use of crutches along with orthoses in order to take some stress off the legs [68]. and standing frames are also used to help children with more severe limitations bear weight through their legs and maintain a full range of motion at all lower limb joints [52]. Furthermore, some children may require casting as a way of treating and preventing contractures. Casting aims to develop a gradual increase in the range of motion available at a certain joint and is a very effective method of improving range of motion at tight joints without the use of surgery [69]. Other children may benefit from the use of a wheelchair, as it can give them more freedom of movement if their walking is limited and strenuous. This can be alternated with the use of orthosis for shorter distances. A wheelchair can also help children keep pace with other able-bodied people, and enable them to participate in recreational activities at school [52].
- Parent/carer education
- Physiotherapy management will eventually be handed over to the parents or carers of the infant. Initially they will be encouraged to observe the physiotherapist carrying out ra ange of motion exercises and handling and positioning strategies before being asked to duplicate these treatments independently. Following these teaching sessions, certain roles will then be handed over to the parents and carers. Following discharge home and as the child begins to mobilise more independently the parents and carers should actively become involved in assessing their child’s progression through observations at home when playing, sitting, crawling etc. This can help with early identification of any differences in the child’s movements or sitting postures between the home and the hospital. It may also allow other possible problems to be identified early on so that a management strategy may be developed. This is essential particularly later on when the child becomes more medically stable, as they will not receive as much medical input and interaction as when the child was a new orn infant in hospital [52]
- The physiotherapist, along with other members of the healthcare team, will be able to offer advice and help parents and carers build confidence in their ability to manage their child’s daily routine [52]
5-Erbs Palsy[edit | edit source]
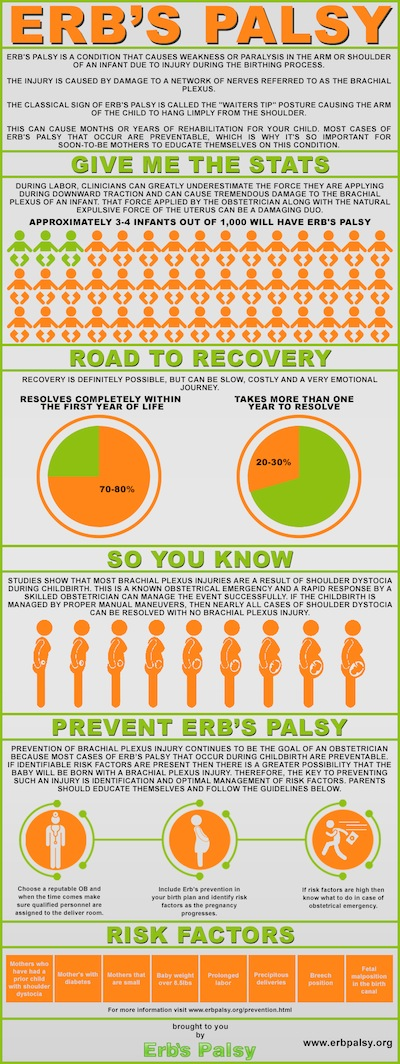
Brachial Plexus Birth Palsy (BPBP) is also commonly known as Obstetric Brachial Plexus Injury (OBBI) and includes Erb’s Palsy, Klumpke’s Palsy and Erb-Klumpke Palsy.
BPBP occurs through damage to the brachial plexus, a grouping of nerves in the shoulder, during birth. Common causes are believed to be shoulder dystocia, excessive or misdirected traction and hyperextension of the arms during birth[70][71]. A recent study shows that it is impossible to predict BPBP[72].
Nerves coming from the brachial plexus supply the muscles, skin and other tissue of the arm and shoulder. Injuries to the brachial plexus disrupt communication from the arm to the brain[70]. This results in a loss or in altered sensation and loss of muscle function. It is common to see the arm of patients with BPBP to hold thir arm in close to their body with it turned inwards. This is sometimes described as the 'Policemans tip'[71].
Birth Brachial Plexus Palsy occurs in between 1.6-2.9 per 1000 live births in developed countries ([73]).
The earlier the signs of recovery the better the prognosis. The return of the function of the biceps muscle in the arm is a key indicator. 95% of infants born with BPBP recover complete function of their arm within 6-12 months while carrying out physiotherapy [71]. However it ,is important to understand that each injury is different and that there is a possibility of a lasting disability with BPBP.
Physiotherapy Management[edit | edit source]
Initial treatment in the first 1-2 weeks after birth will consist of[74][75][76]:
- Careful handling is required and extremes of motion are to be avoided for the first 1 to 2 weeks to allow for the initial inflammatory response to the injury to calm.
- Avoid picking a child up by the arm or from under the armpit. This can compress or stretch the brachial plexus and cause further injury
- Placing a child on their back or in side-lying, with affected limb up, to avoid compression of the injured limb
- Place the affected arm into sleeves before the unaffected arm. This will help avoid extreme movement at the shoulder and will help make dressing quicker and easier.
Encourage parents to carry out specific exercises with their child 2-3 a day in the comfort of their own home - although the exercises can be carried out anywhere appropriate and comfortable. The Home Exercise Programme may focus on the following[74][75][76]:
- Maintain movement at the joints – Ensuring that the joints of the affected limb, especially the shoulder, keep their full range of movement and avoid excessive shortening of the muscles, also called a contracture. This will include passive, assisted and active exercises.
- Increasing the strength of muscles in the affected limb.
- Increasing the child’s awareness of the arm through tactile touch and contact.
- Teaching parents, carers and the child how to handle the affected limb and how to position it for both comforts, prevention of complications and practicality.
- The use of Constraint-Induced Movement Therapy (CIMT) and bimanual/bilateral therapy are sometimes also considered by Physiotherapists.
Surgical Management [edit | edit source]
Surgical intervention is a possible treatment option and will be considered by the medical team after appropriate assessment[77][71]. Surgery is only considered when conservative treatment (such as physiotherapy) is deemed unsuitable[70]. This may be just after birth, as the severity of the BPBP injury requires surgical intervention, or it may be later in a child’s development. Surgery for BPBP can involve nerve transplants or tendon transfer of functioning muscles[78][79]. Many children show a complete recovery, but for those unfortunate not to recover fully, it is important to focus on helping a child to adapt to tasks and work on different strategies to complete activities in their daily life.
Living with BPBP[edit | edit source]
Dina Shafer, who also has a lasting disability affecting her right arm due to BPBP, has a Youtube channel showing how she carries out her activities of daily living such as driving her car and curling her hair as well as how she exercises at the gym where her workouts involve a range of different exercises from deadlifts to martial arts training.
A number of individuals diagnosed with BPBP went on to have successful careers in their chosen field, Martin Sheen was diagnosed with BPBP as was Adrain Clayborn, an American Football player who was drafted 20th overall in the 2011 Draft by the Tampa Bay Buccaneers.
There are also other inspiring individuals out there, including Emily Langridge, a young woman with BPBP who created a documentary following families, adults and children with the condition. Watch the film on the website of the Brachial Plexus Palsy Project.
Microcephaly[edit | edit source]
Microcephaly is a disorder which is present from birth or could develop within the first 2 years of life. It consists of an infant’s skull and brain not growing adequately[80] and being greatly smaller than the average size of other baby’s heads of the same age and gender[81]. Babies head circumference measurement indicates whether or not a baby’s brain is growing as it should be[82].
Causes[edit | edit source]
The condition may be caused by genetic (hereditary) abnormalities or from foetal exposure to drugs, alcohol, maternal diabetes, certain viruses, or toxins during pregnancy, which can lead to damage to the developing brain[81],[81]. Acquired microcephaly could occur after birth if the baby’s infection is present or the brain becomes starved of oxygen[82]. Some children may have normal intellect and the head that will grow bigger, however they will still grow below the normal head circumference[82].
Signs and Symptoms[edit | edit source]
Symptoms vary with each child depending on the severity of the syndrome, children with microcephaly may have[83],[81],[80]:
- Mental retardation
- Delayed motor functions and speech
- Facial alterations,
- Dwarfism or short stature
- Hyperactivity (abnormally active)
- Seizures
- Difficulties with balance and coordination
- High-pitched cry
- Feeding difficulties
Management[edit | edit source]
Microcephaly is a lifetime condition and some symptoms might become more obvious as the child ages and grows. Physiotherapy management focuses on early childhood intervention programs that may help a child optimise their functional ability via promoting normal development, specific exercises, and provides support to the child as well as their families. This carries on through the child’s adulthood to aid the improvement of quality of life, confidence and to integrate normally at home and in the community[81]. The child’s self-esteem should be strengthened via positive reinforcement to promote as much independence as possible[84]. Furthermore, medications can often be used to manage hyperactivity, control seizures and neuromuscular symptoms. The use of genetic counselling may be appropriate for families understand future risk for microcephaly in later pregnancies[85].
6-Hydrocephalus[edit | edit source]
Hydrocephalus is an abnormal build-up of fluid within and around the brain, which can due to excess fluid production, obstruction to its flow, and inadequate absorption[80] If left untreated, the excess fluid can cause increase the pressure put on the skull and brain, which can be damaging[86]. There are two different types of hydrocephalus, which can affect children, these are congenital and acquired.
Congenital Hydrocephalus[edit | edit source]
Congenital hydrocephalus is present from birth and can be caused when a baby is born prematurely (prior to week 37 of pregnancy). Premature birth can lead to bleeding in the infant’s brain, which could blow the flow of fluid in the brain, leading to enlargement of the brain and skull[87]. It is also known to develop when babies are born with other serious health conditions, such as spina bifida. Furthermore, it can also be caused via the change of a genetic material known as X chromosome, as well as rare genetic disorders (e.g. Danny Walker malformation), and arachnoid cysts, which are fluid- filled sacs found between the spinal cord or the brain, and the arachnoid membrane (one of three membranes surrounding the spinal cord and brain)[88]. However, in many cases the cause of congenital hydrocephalus is unknown[88].
Symptoms[edit | edit source]
Congenital hydrocephalus often has distinct physical features and symptoms, including[89],[90],[87]:
- Abnormally large head
- Tense or bulging fontanelle (the soft spot on top of a baby’s head)
- Thin and shiny scalp with veins easily noticeable
- Leg muscles may seem rigid and be prone to uncontrollable muscle contractions (spasms)
- Eyes appear to be looking down (known as the ‘setting-sun sign’ due to the eyes resembling the sun setting below the horizon)
- Feeding difficulties (at high risk for malnutrition)
- Poor general health- high risk for infections
- Increased irritability
- Increased drowsiness/ lethargic
Acquired Hydrocephalus[edit | edit source]
Acquired hydrocephalus develops in adults or children and usually develops following illness or injury. If the condition progresses, the headaches might become continuous and if left untreated, hydrocephalus can be life-threatening. It is possible that acquired hydrocephalus could be caused in several possible ways, such as bleeding inside the brain (subarachnoid haemorrhage)[88], blood clots inside the blood vessels of the brain (venous thrombosis), an infection of the protective membranes surrounding the spinal cord and the brain (meningitis), brain tumours, head injury, or stroke. Furthermore, it is also possible for a baby to be born with narrowed brain passageways, which restrict brain fluid flow, but no symptoms are caused until several years later[87].
Symptoms[edit | edit source]
Acquired hydrocephalus symptoms include[90],[89]:
- Headaches
- Neck pain
- Feeling and being sick (sometimes worse in the morning)
- Drowsiness (can progress to a coma)
- Mental state changes (confusion)
- Blurred or double vision
- Walking difficulty
- Uncontrollable bladder (urinary incontinence)
- In some cases uncontrollable bowel (bowel incontinence)
Medical Management[edit | edit source]
Babies born or children who have developed with hydrocephalus usually require swift treatment to reduce the pressure on their brain. If the condition is not treated, the increase in pressure will damage the brain. Both congenital and acquired hydrocephalus can be treated with shunt surgery or neuroendoscopy[91].
Shunt Surgery[edit | edit source]
Shunt surgery consists of inserting a thin tube (a shunt) in the brain, which is used to remove excess fluid from the brain. The fluid passes through the shunt to a different body part, usually the abdomen (the belly) area, and is then absorbed into the blood stream. The shunt has a valve for the control fluid flow and to make sure it drains gradually and not too quickly, the valve can be felt as a lump under the skin of the scalp[91],[92]. Shunt surgery is carried out by a specialist in surgery of the brain and nervous system (neurosurgeon). A general anaesthetic will be given before the operation so that the child will be asleep throughout the procedure, which usually takes between one to two hours. After the operation, a child will need to stay in the hospital for a few days to recover. Once the shunt has been fitted, there is a risk that there could be shunt malfunction, such as blockage or infection of the shunt, leading to the need for essential additional treatment[91],[92]. Some symptoms of shunt malfunction are headaches, sickness, confusion, drowsiness, redness, increased temperature, neck stiffness, abdominal pain, and irritability[91].
Endoscopic third ventriculostomy (ETV)[edit | edit source]
ETV involves creating a hole in the bottom of the brain, which allows trapped fluid to discharge to the surface of the brain where it can then be absorbed. However, an ETV is not suitable for everyone, but it may be a possible treatment option if the excess fluid in the brain is resulting from a blockage, known as obstructive hydrocephalus. The fluid will then be able to drain out via the hole and avoiding any blockage from occurring. A general anaesthetic is given prior the operation and the neurosurgeon will then create a small hole the skull of the child and use a small camera device (endoscope) to look inside brain chambers. A small hole will then be created inside the brain with the help of the endoscope. This procedure will take approximately one hour[91],[92].
There is reduced risk of infection following ETV surgery compared with the shunt, but there is still a chance that the ETV’s may block months or years after surgery. However, there are some risks associated with ETV, such as hole closure, inability of the brain to absorb excess fluid, or bleeding in the brain (usually minor)[91]
Physiotherapy Management[edit | edit source]
Regardless of the different surgical management, children with hydrocephalus still have some disabilities. Therefore, early involvement with physiotherapists via different methods of rehabilitation is essential, whether surgical or non-surgical management is required. Additionally, successful shunting is usually related to more obvious and rapid improvements in rehabilitation efforts[93]. Specific treatment procedures are numerous, functional training for activities of daily living; therapeutic exercise; manual techniques such as mobilization and stretching; and therapeutic modalities[94].
Physiotherapy goals are aimed at:
- Improving functional skills and reducing secondary impairment, such as obesity, contractures, and fractures, which could delay developmental skills. Furthermore, physiotherapists can work with children in their home and in the hospital or clinic, depending on their medical conditions and age[95].
- Motor control (co-ordination of muscles and limbs), learning theories, and development are factors that contribute to occurrence of motor behaviour (how the muscles and limbs react to movement, control, development, and learning). These factors include not only the central nervous system (brain and spinal cord) as the driving force, but also biomechanical (human movement principles), psychological, social, and environmental components[94].
- Teaching and practicing skills under these theories is task-oriented (specific to everyday movements e.g. sitting to standing), which should be intermittent and repetitive. A high level of learning occurs via a child’s problem solving instead of by the therapist's hands-on facilitation. It is also important that emphasis is placed on family centred care and treatment in natural environments.
- The mutual goal is usually to increase functional activity, which in turn, decreases disability[94].
These goals should be achieved by[96]:
- Promoting physical milestones of achievement such as sitting, crawling, standing
- Optimising mobility independence
- Improving balance and coordination via exercise
- Stretching tight muscles via exercise
- Strengthening weak muscles via exercise
- Increasing quality of life and confidence
- Improving endurance and exercise tolerance
General Activity Guidelines[edit | edit source]
It is still important to follow certain exercise guidelines, whether a child has a medical condition and/or disability. Children should take part in a variety of activities at moderate to vigorous intensity for at least 60 minutes per day, including weight-bearing activity at least twice weekly. This will provide high physical stresses, which will increase muscle strength, bone health, and flexibility. It is also possible to break down this 60 minute into short, 10-minute sessions and still achieve the same gains[97].
Moderate-intensity physical activity heightens heart and breathing rates to a level the child feels warmer, the pulse can be felt, and may sweat when indoors or on a hot or humid day. Vigorous activity will result in a child being out of breath and/or sweating. Participation in moderate to vigorous activity can range from sport, formal exercise, and other physically demanding exercise (e.g. dancing and swimming) to active play. Activities of daily living, such as walking, climbing stairs and cycling can also give a child some of the 60 minutes of physical activity required[97].
Health practitioners, local authority, and physical activity professionals can make parents and carers aware of the benefits of undertaking 60 minutes of moderate to vigorous physical activity a day. Parents should engage with their children whilst taking part in activity, by either encouraging their child and/or getting involved in activities with the child. This can be achieved by acting as a role model and encourage completing local journeys via physically active modes of travel (e.g. walking and cycling)[98]. If these guidelines are met by children, they are at reduced risk of chronic conditions (e.g. obesity) and their general health and wellbeing will be improved[99].
Keeping Active[edit | edit source]
While staying active is extremely beneficial for young people and adults alike, it is important to remember that a disability should not prevent people from being and staying active and talented individuals should be encouraged to perform to the highest level if that is something they wish to do! The following section provides some information on sporting opportunities for individuals with disabilities in the UK.
Disability Sports[edit | edit source]
| Alpine Skiing | Equestrian | Shooting |
| Archery | Football 5-a-side | Wheelchair basketball |
| Badminton | Goalball | Wheelchair curling |
| IPC Biathlon | Ice Sledge Hockey | Wheelchair Dance Sport |
| Boccia | Judo | Wheelchair Fencing |
| Canoe | Powerlifting | Wheelchair rugby |
| Cross-Country skiing | Rowing | Wheelchair tennis |
| Cycling | Sailing | |
More information on the details of each sport can be found on the website of the Paralympic Movement and also in sport-specific fact sheets created by the Scottish Disability Sport , the governing body for sports for people of all ages and abilities with a physical, sensory or learning disability.
There are a number of different organisations who are involved in disability sport throughout the UK.
Associations representing different disabilities and impairments:
- British Amputee & Les Autres Sports Association - https://sites.google.com/a/balasa.org.uk/main/
- British Blind Sport - http://www.britishblindsport.org.uk/
- Cerebral Palsy Sport - http://www.cpsport.org/
- Dwarf Sport UK - http://www.dsauk.org/
Other organisations include:
- Mencap an oragnisation that supports people with learning disabilities to live the life they choose. They have numerous projects, some of which are activated and sport-related. More information can be found at http://www.mencap.org.uk/
- Special Olympics Great Britain provide year-round training and competition for children and adults with learning disabilities encouraging physical fitness, inclusion, development of social skills and the building of friendships - http://www.specialolympicsgb.org.uk/
- Deaf Sports UK looks to encourage Deaf people to participate, enjoy and excel at sport. http://www.ukdeafsport.org.uk/
- Wheel Power - http://www.wheelpower.org.uk/WPower/
Paralympics[edit | edit source]
The British Paralympic Association are responsible for selecting, preparing, funding and managing the athletes representing Great Britain and Northern Ireland in the Paralympics. ParalympicsGB has been extremely successful, coming in top of three of the last four summer games and maintain success on the field, in a range of different sports as one of their main priorities. The association believes that developing and showcasing the Paralympics will help shift perceptions of disability sport and disabled people across the globe.
The association’s current vision for 2012-2017 is:
“Through sport, inspire a better world for disabled people”
Their mission is: “To make the UK the leading nation in Paralympic sport” with regard to performance on the field of play, support for athletes, advocacy and influence, promotion of disability sport and development of opportunities and participation at a grassroots level.
Find out more at: http://www.paralympics.org.uk
The Paralympic Games[edit | edit source]
The first Paralympic Games were held in Rome, Italy in 1960, featuring 400 athletes from 23 countries, competing in 13 sports. The London 2012 Paralympic Games featured more than 4250 athletes from 164 countries taking part in 20 different sports!
The International Paralmypic Committee (IPC)[edit | edit source]
The vision of the IPC, founded as a non-profit organisation in 1989, is “To enable Paralympic athletes to achieve sporting excellence and inspire and excite the world” with its core values outlined as Courage, Determination, Inspiration and Equality. The IPC developed the Paralympic Games to showcase the achievements of athletes with impairments to a global audience in order to change societal perceptions and create lasting legacies.
More information on the IPC can be found at http://www.paralympic.org/
Eligible Impairments[edit | edit source]
The Paralympic movement recognises 10 different impairment types as eligible for participation in the Games, these include:
- Impaired Muscle Power
- Impaired Passive Range of Motion
- Loss of limb or limb deficiency
- Leg-length difference
- Short stature
- Hypertonia
- Ataxia
- Athetosis
- Impairment of the eye, optic nerve or visual cortex
- Intellectual impairment
Get Set[edit | edit source]
Get Set is the official youth engagement programme of the British Paralympic Association and the British Olympic Association. The programme is aimed at inspiring and developing learning opportunities around the Paralympic and Olympic games.
The programme aims to:
- Give all young people the chance to learn about and live the Olympic Values of friendship, excellence and respect and the Paralympic Values of inspiration, determination, courage and equality
- Build excitement about Team GB and ParalympicsGB, using the Olympic and Paralympic Games as a hook for learning and participation
Get Set has resources such as lesson ideas, whole school activities, assemblies and athlete stories, films and images that can be used to engage young people. The programme also will be encouraging young people to complete challenges related to the Paralympic and Olympic games and will be challenging them to ‘travel’ the distance to Rio De Janeiro with their Road To Rio resources!
For more information access the main Get Set website at http://www.getset.co.uk
Information on Paralympians including biographies, blogs, features and interviews can be found at http://www.paralympic.org/athletes
Stories behind the conditions[edit | edit source]
|
A parent of a child with Spina Bifida tells us her story |
|
Extraordinary story of Ben Jackson who was born with cerebral palsy but refuses to let it get in the way of his dream to be a competitive wrestler |
|
This is the story of Harrison Smith who was born with Duchenne Muscular Dystrophy in January 2011 |
Equipment and Aids[edit | edit source]
There is lots of physiotherapy equipment available to support children with Neurological conditions.
- Mobility and standing aids: this equipment is designed to assist with walking or getting around. This may include walkers, crutches and wheelchairs.
- Postural management: mostly seating and sleeping equipment which helps to keep children in good posture and positions to help prevent discomfort, contractures and deformities.
- Equipment for house and home: this equipment is designed to help getting around during daily living activities such as getting out of bed. It can may include grab rails, hoists and transfer aids.
- Play and development: children learn through play and there are lots of adapted toys which can help develop children’s skills and abilities whilst having fun.
- Orthotics and splints: These are braces worn mostly on the arms and legs. They can help prevent deformities, improve walking and control and relieve pressure. They can be functional or accommodative.
There is lots of specialised equipment and methods specifically designed to support CP children, and allowing them to be as independent as possible as well as equipment to help the caregiver to look after these children. Some of this equipment include:
- Special designed clothing for CP children: simplified clothing to make dressing easier.
- Devices to assist with eating and drinking, e.g. eating utensils and bottles
- Bathing items to improve safety and assist with personal hygiene and personal care
- Adapted car seats and vehicles.
Normally an occupational therapist will assess and provide this type of equipment. Physiotherapists and occupational therapists often work together when deciding on what equipment may be best for a child.
References[edit | edit source]
- ↑ Dolk H, Loane M, Garne E. The prevalence of congenital anomalies in Europe. Advances in experimental medicine and biology. 2010;686:349-64.
- ↑ RITTER, T. (1998) Children with cerebral palsy: a parent's guide. Ed Elaine Geralis; Woodbine House
- ↑ CEREBRAL PALSY. 2013. [online].[viewed 10 November 2014]. Available from: http://cerebralpalsy.org/
- ↑ 4.0 4.1 4.2 4.3 4.4 4.5 TECKLIN, J.S., 2006. Pediatric physical therapy / [editied by] Jan S. Tecklin. Philadelphia : Lippincott Williams and Wilkins, 2008; 4th ed.
- ↑ 5.0 5.1 5.2 NHS., 2013. Muscular Dystrophy [online]. [viewed 3 October 2014]. Available from: http://www.nhs.uk/conditions/muscular-dystrophy/Pages/Introduction.aspx
- ↑ 6.0 6.1 MEDLINEPLUS., 2014. Duchenne Muscular Dystrophy [online]. [viewed 3 October 2014]. Available from: http://www.nlm.nih.gov/medlineplus/ency/article/000705.htm
- ↑ CENTERS FOR DISEASE CONTROL AND PREVENTION., 2014. Facts About Muscular Dystrophy [online]. [viewed 2 October 2014]. Available from: http://www.cdc.gov/ncbddd/musculardystrophy/facts.html
- ↑ ORPHANET., 2007. Duchenne and Becker Muscular Dystrophy [online]. [viewed 6 October 2014]. Available from: http://www.orpha.net/consor/cgi-bin/OC_Exp.php?Lng=EN&Expert=262
- ↑ FSH SOCIETY: FACIOSCAPULOJUMERAL MUSCULAR DYSTROPHY., 2010. About FSHD [online]. [viewed 5 October 2014]. Available from: https://www.fshsociety.org
- ↑ NHS., 2013. Muscular Dystrophy – Types [online]. [viewed 3 October 2014]. Available from: http://www.nhs.uk/Conditions/Muscular-dystrophy/Pages/Symptoms.aspx
- ↑ SUOMINEN, T., BACHINSKI, L., AUVINEN, S., HACKMAN, P., BAGGERLY, K., ANGELINI, C., PELTONEN, L., KRAHE, R. and UDD, B., July 2011. Population frquency of myotonic dystrophy: higher than expected frequency of myotonic dystrophy type 2 (DM2) mutation in Finland.vol. 19, no. 7 pp. 776-82 Available from: http://www.ncbi.nlm.nih.gov/pubmed/21364698
- ↑ EDWARDS, J.H., 1986. The population genetics of Duchenne: natural and artificial selection in Duchenne muscular dystrophy. Journal of Medical Genetics. Dec, vol. 23, no. 6, pp. 521-530.
- ↑ MDA: FIGHTING MUSCLE DISEASE., 2014. Duchenne Musuclar Dystrophy: Causes / Inheritance [online]. [viewed 7 October 2014]. Available from: http://mda.org/disease/duchenne-muscular-dystrophy/causes-inheritance
- ↑ 14.0 14.1 14.2 14.3 MEDSCAPE., 2014. Muscular Dystrophy [online]. [viewed 5 October 2014]. Available from: http://emedicine.medscape.com/article/1259041-overview#a0112
- ↑ NATIONAL HUMAN GENOME RESEARCH INSTITUE., 2013. Learning About Duchenne Muscular Dystrophy [online]. [viewed 5 October 2014]. Available from: http://www.genome.gov/19518854
- ↑ ALLEN, K. Duchenne Muscular Dystrophy. HealthandFitnessTalk.com. July 10, 2013, Available from: http://www.healthandfitnesstalk.com/tag/duchenne-muscular-distrophy/
- ↑ 17.0 17.1 MEDLINEPLUS., 2014. Contracture deformity [online]. [viewed 2 October 2014]. Available from: http://www.nlm.nih.gov/medlineplus/ency/article/003185.htm
- ↑ 18.0 18.1 HYDE, S.A., FLØYTRUP, I., GLENT, S., KROKSMARK, A., SALLING, B., STEFFENSEN, B.F., WERLAUFF, U. and ERLANDSEN, M., 2000. A randomized comparative study of two methods for controlling Tendo Achilles contracture in Duchenne muscular dystrophy. Neuromuscular Disorders. , vol. 10, no. 4, pp. 257-263.
- ↑ 19.0 19.1 19.2 WALLACE, G.B. and NEWTON, R.W., 1989. Gowers' sign revisited. Archives of Disease in Childhood. Sep, vol. 64, no. 9, pp. 1317-1319.
- ↑ LEIBOWITZ, D. and DUBOWITZ, V., 1981. Intellect and behaviour in Duchenne muscular dystrophy. Developmental Medicine & Child Neurology. , vol. 23, no. 6, pp. 577-590
- ↑ 21.0 21.1 CYRULNIK, S.E., FEE, R.J., DE VIVO, D.C., GOLDSTEIN, E. and HINTON, V.J., 2007. Delayed developmental language milestones in children with Duchenne’s muscular dystrophy. The Journal of Pediatrics. , vol. 150, no. 5, pp. 474-478.
- ↑ PROSENSA., 2014. DMD - Duchenne Muscular Dystrophy [online]. [viewed 3 October 2014]. Available from: http://www.prosensa.eu/hc-professionals/duchenne-muscular-dystrophy
- ↑ LORD, J., BEHRMAN, B., VARZOS, N., COOPER, D., LIEBERMAN, J.S. and FOWLER, W.M., 1990. Scoliosis associated with Duchenne muscular dystrophy. Archives of Physical Medicine and Rehabilitation. Jan, vol. 71, no. 1, pp. 13-17.
- ↑ PT PRODUCTS., 2006. Continuing to Stand Tall [online]. [viewed 8 October 2014]. Available from: http://www.ptproductsonline.com/2006/10/continuing-to-stand-tall/
- ↑ GARRALDA, M.E., MUNTONI, F., CUNNIFF, A. and CANEJA, A.D., 2006. Knee–ankle–foot orthosis in children with duchenne muscular dystrophy: User views and adjustment. European Journal of Paediatric Neurology. , vol. 10, no. 4, pp. 186-191.
- ↑ STEVENS, P.M. Lower Limb Orthotic Management of Duchenne Muscular Dystrophy: A Literature Review. American Academy of Orthotists & Prothetists2006, vol. 18, no. 4 pp. 111-119 Available from: http://www.oandp.org/jpo/library/2006_04_111.asp
- ↑ MUNTONI, F., FISHER, I., MORGAN, J.E. and ABRAHAM, D., 2002. Steroids in Duchenne muscular dystrophy: from clinical trials to genomic research. Neuromuscular Disorders. , vol. 12, pp. S162-S165.
- ↑ 28.0 28.1 ANGELINI, C. and PETERLE, E., 2012. Old and new therapeutic developments in steroid treatment in Duchenne muscular dystrophy. Acta Myologica : Myopathies and Cardiomyopathies : Official Journal of the Mediterranean Society of Myology / Edited by the Gaetano Conte Academy for the Study of Striated Muscle Diseases. May, vol. 31, no. 1, pp. 9-15.
- ↑ OGATA, H., ISHIKAWA, Y., ISHIKAWA, Y. and MINAMI, R., 2009. Beneficial effects of beta-blockers and angiotensin-converting enzyme inhibitors in Duchenne muscular dystrophy. Journal of Cardiology. , vol. 53, no. 1, pp. 72-78.
- ↑ MATSUMURA, T., 2014. Beta-blockers in Children with Duchenne Cardiomyopathy. Reviews on Recent Clinical Trials. , vol. 9, no. 2, pp. 76-81.
- ↑ GREAT ORMOND STREET HOSPITAL CHILDREN’S CHARITY., 2012. Duchenne muscular dystrophy and surgery [online]. [viewed October 7 2014]. Available from: http://www.gosh.nhs.uk/medical-information/procedures-and-treatments/duchenne-muscular-dystrophy-and-surgery/
- ↑ SETHNA, N.F., ROCKOFF, M.A., WORTHEN, H.M. and ROSNOW, J.M., 1988. Anesthesia-related complications in children with Duchenne muscular dystrophy. Anesthesiology. , vol. 68, no. 3, pp. 462-464.
- ↑ GALASKO, C.S., 1993. Medical management of Duchenne muscular dystrophy. BMJ (Clinical Research Ed.). Mar 27, vol. 306, no. 6881, pp. 859.
- ↑ KAROL, L.A., 2007. Scoliosis in patients with Duchenne muscular dystrophy. The Journal of Bone & Joint Surgery. , vol. 89, no. suppl_1, pp. 155-162
- ↑ BUSHBY, K., FINKEL, R., BIRNKRANT, D.J., CASE, L.E., CLEMENS, P.R., CRIPE, L., KAUL, A., KINNETT, K., MCDONALD, C. and PANDYA, S., 2010. Diagnosis and management of Duchenne muscular dystrophy, part 2: implementation of multidisciplinary care. The Lancet Neurology. , vol. 9, no. 2, pp. 177-189.
- ↑ 36.0 36.1 36.2 DI MARCO, M., YIRRELL, J., TEWNION, J., KEDDIE, A., MELVILLE, P. and HARRISON, L. DMD Scottish Physiotherapy Managment Profile. Available from: http://www.smn.scot.nhs.uk/DMD%20management%20profile%20-%20version%2020b).pdf
- ↑ MUSCULAR DYSTROPHY CAMPAIGN., 2009. A Home Exercise Book: Physiotherapy management for Duchenne muscular dystrophy [online]. [viewed 7 October 2014]. Available from: http://www.muscular-dystrophy.org/assets/0001/1477/Physio_booklet_web.pdf
- ↑ 38.0 38.1 MAZZONE, E., MESSINA, S., VASCO, G., MAIN, M., EAGLE, M., D’AMICO, A., DOGLIO, L., POLITANO, L., CAVALLARO, F. and FROSINI, S., 2009. Reliability of the North Star Ambulatory Assessment in a multicentric setting. Neuromuscular Disorders. , vol. 19, no. 7, pp. 458-461.
- ↑ MUSCULAR DYSTROPHY CAMPAIGN. North Star Ambulatory Assessment [online]. [viewed 8 October 2014]. Available from: http://www.muscular-dystrophy.org/assets/0000/6388/NorthStar.pdf
- ↑ 40.0 40.1 40.2 REILLY, M., MURPHY, S. and LAURA, M., 2011. Charcot-Marie-Tooth disease. Journal of the Peripheral Nervous System. vol. 16, pp. 1-14
- ↑ 41.0 41.1 41.2 41.3 PAREYSON, D. and MARCHESI, C., 2009. Diagnosis, natural history, and management of Charcot-Marie-Tooth disease. Lancet Neural. vol. 8, pp. 654-657.
- ↑ LAFARGE, C., TALSANIA, K., TOWNSHEND, J. and FOX, P. 2014. Living with Charcot-Marie-Tooth Disease: a qualitative analysis. British Journal of Neuroscience Nursing. October/November, vol. 10, no. 5, pp. 226-235.
- ↑ NHS CHOICES., 2014. Charcot-Marie-Tooth disease-Causes [online]. [viewed 9 November 2014]. Available from: http://www.nhs.uk/Conditions/Charcot-Marie-Tooth-disease/Pages/Causes.aspx
- ↑ 44.0 44.1 PHYSIO.CO.UK., Charcot Marie Tooth [online]. [viewed 10 November 2014]. Available from: http://www.physio.co.uk/what-we-treat/neurological/conditions/charcot-marie-tooth.html
- ↑ CMT UNITED KINGDOM., 2013. Symptoms/Problems [online]. [viewed 10 November 2014]. Available from: http://www.cmt.org.uk/symptoms_problems.php
- ↑ 46.0 46.1 MUSCULAR DYSTROPHY ASSOCIATION., 2009. Facts About Charcot-Marie-Tooth Disease & Related Diseases. [online]. [viewed 8 November 2014]. Available from: http://mda.org/sites/default/files/publications/Facts_CMT_P-180_0.pdf
- ↑ THOMAS, F.P., GUERGUELTCHEVA, V., GONDIM, F. and JORDANOVA, A., 2014.Charcot-Marie-Tooth Diseases. In: B. Katirji ed. Neuromuscular Disorders in Clinical Practice.New York: Springer Science + Business, pp. 519-547.
- ↑ NHS CHOICES., 2014. Charcot-Marie-Tooth disease- Treatment [online]. [viewed 9 November 2014]. Available from: http://www.nhs.uk/Conditions/Charcot-Marie-Tooth-disease/Pages/Treatment.aspx
- ↑ 49.0 49.1 49.2 CMT UNITED KINGDOM., 2013. Exercise/Physiotherapy [online]. [viewed 10 November 2014]. Available from: http://www.cmt.org.uk/exercise-physiotherapy.php
- ↑ 50.0 50.1 GRANDIS, M. and SHY, M. 2005. Current Therapy for Charcot-Marie-Tooth Disease. Current Treatment Options in Neurology. vol. 7, no. 1, pp. 23-31.
- ↑ NARDONE, A., GODI, M., ARTUSO, A. and SCHIEPATTI, M. 2010. Balance Rehabilitation by Moving Platform and Exercises in Patients with Neuropathy or Vestibular Deficit. Archives of Physical Medicine and Rehabilitation. vol. 91, pp. 1869-1877.
- ↑ 52.00 52.01 52.02 52.03 52.04 52.05 52.06 52.07 52.08 52.09 52.10 52.11 TAPPIT-EMAS, E., 2008. Spina Bifida. In J.S, TECKLIN, 4TH eds. Pediatric Physical Therapy. Phyiladelphia: Wolters Kluwer & Lippincott Williams and Wilkins, pp. 231-280
- ↑ 53.0 53.1 53.2 53.3 53.4 SANDLER., A.D., 2010. Children with Spina Bifida: Key Clinical Issues. Pediatr Clin N Am. Vol. 57, pp. 879-892.
- ↑ RAY, J.G., MEIER, C., VERLEULEN, M.J., BOSS, S., WYATT, P.R. & COLE, D.E.C., 2002. Association of neural tube defects and folic acid food fortification in Canada. Lancet. Vol. 360 (9350), pp. 2047-2048.
- ↑ BARF, H.A., VERHOEF, M., JENNEKENS-SCHINKEL, A., POST, M.W.M., GOOSHKENS, R.H.J.M. & PREVE, A.J.H., 2003. Cognitive status of young adults with spina bifida. Dev Med Child Neurol. Vol. 45, pp. 813-20.
- ↑ HINDERER, K.A., HINDERER, S.R. & SHURTLEFF, D.B., 2006. Myelodysplasia. In CAMPBELL, S.K., VANDER LINDEN, D.W. & PALISANO, R.J. Physical Therapy for Children. 3rd edition. Pp. 735-789. Philadelphia: Saunders Elsevier
- ↑ 57.0 57.1 PARK BROWN, J., 2001. Orthopaedic care of children with Spina Bifida: You’ve come a long way, baby! Orthopaedic Nursing. Vol. 20 (4), pp. 51-58
- ↑ AUSILI, E., FOCARELLI, B., TABACCO, F., FORTUNELLI, G., CARADONNA., P., MASSIMI, L., SIGISMONDI, M., SALVAGGIO, E. & RENDELI,C., 2008. Bone mineral density and body composition in a myelomeningocoele children population: effects of walking ability and sport activity. European Review for Medical and Pharmacological Sciences. Vol. 12, pp. 349-354
- ↑ LAPIDES, J., DIOKNO, A.C., SILBER, S.J., ET AL 1972. Clean, intermittent self-catheterisation in the treatment of urinary tract disease. J Urol. Vol. 107, pp. 458-61.
- ↑ LEIBOLD, S., 2008. Neurogenic bowel and continence programmes for the individual with spina bifida. J Pediatr Rehabil Med. Vol. 1, pp. 325-36
- ↑ SWANK., M. & DIAS., L., 1992. Myelomenignocoele: a review of the orthopaedic aspects of 206 patients treated from birth with no selection criteria. Dev Med Child Neurol. Vol. 34, pp. 1047-52
- ↑ O’CONNELL, D.G. & BARNHART, R., 1995. Improvement in wheelchair propulsion in pediatric wheelchair users through resistance training: A pilot study. Archives of Physical Medicine and Rehabilitation. Vol. 76, pp. 368-372
- ↑ ANDRADE, C.K., KRAMER, J., GARBER, M. & LONGMUIR, P., 1991. Changes in self concept, cardiovascular endurance and muscular strength of children with spina bifida aged 8 to 13 years in response to a 10-week physical-activity programme: A pilot study. Child: Care Health and Development. Vol. 17, pp. 183-196
- ↑ THOMPSON, D.N.P., 2009. Postnatal management and outcome for neural tube defects including spina bifida and encephalocoeles. Prenat Diagn. Vol. 29, pp. 412-419
- ↑ SEITZBERG, A., LIND, M. & BIERING-SORENSEN, F., 2008. Ambulation in adults with myelomeningocoele. Is it possible to predict the level of ambulation in early life? Childs Nerv Syst. Vol. 24 (2), pp. 231-237
- ↑ 66.0 66.1 CUDDEFORD, T.J., FREELING, R.P., THOMAS, S.S., AIONA, M.D., REX, D., SIROLLI, H. & ELLIOT, J., 1997. Energy consumption in children with myelomeningocoele: a comparison between reciprocating gait orthosis and hip-knee-ankle-foot orthosis ambulators. Developmental Medicine & Child Neurology. Vol. 39, pp. 239-242.
- ↑ YNGVE, D.A., DOUG, A.S.R. & ROBERTS, J.M., 1986. The reciprocating gait orthosis in myelomeningocoele. Journal of Pediatric Orthopaedics. Vol. 4, pp. 304-310.
- ↑ MAZUR, J.M. & KYLE, S., 2004. Efficacy of bracing the lower limbs and ambulation training in children with myelomeningocoele. Developmental Medicine & Child Neurology. Vol. 46, pp. 352-356
- ↑ AL ORAIBI, S., 2014. Non-surgical intervention of knee flexion contracture in children with spina bifida: case report. J Phys Ther Sci. Vol. 26, pp. 793-795
- ↑ 70.0 70.1 70.2 ZAFEIRIOU, D.I. and PSYCHOGIOU, K., 2008. Obstetrical brachial plexus palsy. Pediatr Neurol. Vol. 38, no. 4, pp. 235-242
- ↑ 71.0 71.1 71.2 71.3 YANG, L.J.S., 2014. Neonatal brachial plexus palsy—Management and prognostic factors. Seminars in Perinatology. Vol. 38, no. 4, pp. 222-234
- ↑ OUZOUNIAN, J.G., 2014. Risk factors for neonatal brachial plexus palsy. Seminars in Perinatology. Vol. 38, no. 4, pp. 219-221
- ↑ PONDAAG, W., MALESSY, M.J.A., VAN DIJK, J.G. and THOMEER, R.T.W.M., 2004. Natural history of obstetric brachial plexus palsy: a systematic review. Developmental Medicine & Child Neurology. Vol. 46, no. 2, pp. 138-144.
- ↑ 74.0 74.1 CLARKE, H.M. and CURTIS, C.G., 1995. An approach to obstetrical brachial plexus injuries. Hand Clin. Vol. 11, no. 4, pp. 563-580; discussion 580-561
- ↑ 75.0 75.1 SHENAQ, S.M., BULLOCKS, J.M., DHILLON, G., LEE, R.T. and LAURENT, J.P., 2005. Management of infant brachial plexus injuries. Clinics in Plastic Surgery. Vol. 32, no. 1, pp. 79-98
- ↑ 76.0 76.1 WATERS, P.M., 2005. Update on management of pediatric brachial plexus palsy. J Pediatr Orthop B. Vol. 14, no. 4, pp. 233-244
- ↑ HALE, H.B., BAE, D.S. and WATERS, P.M., 2010. Current concepts in the management of brachial plexus birth palsy. The Journal of hand surgery. Vol. 35, no. 2, pp. 322-331
- ↑ HALE, H.B., BAE, D.S. and WATERS, P.M., 2010. Current concepts in the management of brachial plexus birth palsy. The Journal of hand surgery. Vol. 35, no. 2, pp. 322-331
- ↑ BIRCH, R. 2011.Birth lesions of the brachial plexus.In:ed. Surgical disorders of the peripheral nerves, Springer, 429-481
- ↑ 80.0 80.1 80.2 SHEPHERD, R.B., 1995. Physiotherapy in Paediatrics. 3rd ed. Oxford: Butterworth Heinemann.
- ↑ 81.0 81.1 81.2 81.3 81.4 YORKSHIRES CHILDREN’S PHYSIOTHERAPY., 2014. [online]. [viewed 13 October 2014] Available from: http://www.yorkshirechildrensphysiotherapy.co.uk/conditions-treated/congenital-and-acquired-neuromuscular-and-genetic-disorders/
- ↑ 82.0 82.1 82.2 HEALTHLINE., 2014. [online]. [viewed 23 October 2014] Available from: http://www.healthline.com/symptom/microcephaly
- ↑ MEDICINENET., 2014. [online]. [viewed 23 October 2014] Available from: http://www.medicinenet.com/microcephaly/page2.htm#what_are_the_signs_and_symptoms_of_microcephaly
- ↑ CLEVELAND CLINIC CHILDREN’S., 2011. [online]. [viewed 15 October 2014] Available from: http://my.clevelandclinic.org/childrens-hospital/health-info/diseases-conditions/hic-Microcephaly
- ↑ MEDICINENET., 2014. [online]. [viewed 13 October 2014] Available from: http://www.medicinenet.com/microcephaly/page3.htm
- ↑ SOCCI, D.J., BJUGSTAD, K.B., JONES, H.C., PATTISAPU, J.V. and ARENDASH, G.W., 1999. Evidence that oxidative stress is associated with the pathophysiology of inherited hydrocephalus in the h-tx rat model. Experimental Neurology. January, vol. 155, no. 1, pp. 109–117.
- ↑ 87.0 87.1 87.2 SCHRANDER-STUMPEL, C. and FRYNS, J.P, 1998. Congenital hydrocephalus: Nosology and guidelines for clinical approach and genetic counselling, European Journal of Paediatrics. May, vol. 157, no. 5, pp. 355-362.
- ↑ 88.0 88.1 88.2 NHS CHOICES., 2014. [online]. [viewed 13 October 2014] Available from: http://www.nhs.uk/Conditions/Hydrocephalus/Pages/Causes.aspx
- ↑ 89.0 89.1 MAYO CLINIC., 2014. [online]. [viewed 17 October 2014] Available from: http://www.mayoclinic.org/diseases-conditions/hydrocephalus/basics/symptoms/con-20030706
- ↑ 90.0 90.1 NHS CHOICES., 2014. [online]. [viewed 13 October 2014] Available from: http://www.nhs.uk/Conditions/Hydrocephalus/Pages/Symptoms.aspx
- ↑ 91.0 91.1 91.2 91.3 91.4 91.5 NHS CHOICES., 2014. [viewed 15 October 2014] Available from: http://www.nhs.uk/Conditions/Hydrocephalus/Pages/Treatment.aspx
- ↑ 92.0 92.1 92.2 MAYO CLINIC., 2014. [online]. [viewed 17 October 2014] Available from: http://www.mayoclinic.org/diseases-conditions/hydrocephalus/basics/treatment/con-20030706
- ↑ BONTKE, C.F., ZASLER, N.D. and BOAKE, C. 1996. Rehabilitation of the head-injured patient. New York, NY: McGraw-Hill.
- ↑ 94.0 94.1 94.2 INSTEP PHYSICAL THERAPY., 2014. [viewed 15 October 2014] Available from: http://www.instepphysio.ca/Neurorehabilitation/Neurorehabilitation/a~3553--c~343920/article.html
- ↑ KARIMZADEH, P. 2012. Management of hydrocephalus. Hydrocephalus [online]. Available from: http://www.intechopen.com/books/hydrocephalus/management-of-hydrocephalus-
- ↑ MANCHESTER PHYSIO., 2014 [viewed 15 October 2014] Available from: http://www.manchesterphysio.co.uk/what-we-treat/paediatric-physiotherapy/hydrocephalus.html
- ↑ 97.0 97.1 NICE, Promoting physical activity for children and young people. 2009. [online]. [viewed 14 November 2014] Available from: http://www.nice.org.uk/guidance/ph17/chapter/1-recommendations#definitions
- ↑ NICE, Promoting physical activity for children and young people. 2009. [online]. [viewed 14 November 2014] Available from: http://www.nice.org.uk/guidance/ph17/chapter/1-recommendations
- ↑ NICE, Promoting physical activity for children and young people. 2009. [online]. [viewed 14 November 2014] Available from: http://www.nice.org.uk/guidance/ph17/chapter/2-public-health-need-and-practice