Clostridium Difficile Infection CDI
Original Editors - John Hardy from Bellarmine University's Pathophysiology of Complex Patient Problems project.
Top Contributors - John Hardy, Lucinda hampton, Reem Ramadan, Elaine Lonnemann, 127.0.0.1, WikiSysop, Oyemi Sillo, Kim Jackson, Vidya Acharya and Nupur Smit Shah
Definition/Description[edit | edit source]
Clostridium difficile is an anerobic, gram-positive bacillus. This rode shaped bacterium exists in vegetative or spore form and can survive harsh environments and common sterilization techniques. Clostridium difficile is resistant to ultra-violent light, high temperatures, and antibiotics. There are two distinct toxins which are the primary virulent factors for C. difficile: toxin A (Tcd A) and toxin B (Tcd B). Both toxins disrupt the acti cytoskeleton of tissue cells by preventing the activation of specific proteins (GTPases, Rho, Rac) that regulate actin polymerization. Originally, TcdA was believed to be the major virulence factor of C. Difficile, but recent evidence supports Tcd B as the primary factor not Tcd A. Approximately 70% of healthy newborns have detectable C. Difficile colonization by 18months of age. These infants rarely develop colitis and it is believed this asymptomatic colonization helps the body develop humoral immunity.[1][2][3][4]
C. Diffile spores are ingested following contact with contaminated abiotic or biotic surfaces. C. Difficile has a propensity to colonize in the large bowel likely due to the anerobic environment and absence of native flora which is often depleted by antibiotic treatment. Bile salts are then utilized for spore germination. Following germination, the vegetative-cell type colonizes the host and begins to produce toxins. These toxins are then released causing cytotoxicity, tissue necrosis, inflammation, fluid secretion, and ultimately apoptosis of epithelial cells.[4][3]
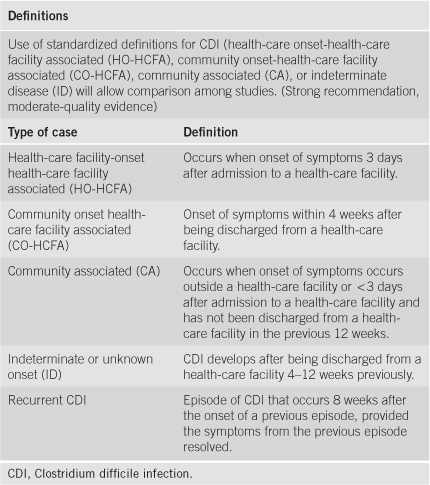
CDI Definitions[3]
Prevalence[edit | edit source]
C. Difficile is commonly a nosocomial pathogen found in a hospital or similar healthcare facility. Colonization rate of healthy adults is estimated to be 2% while the prevalence of hospitalized adults can be high as 40%. Nearly a third of all C. Difficile infections are community acquired which suggests a recent increase in non-nosocmial infections. Risk factors for the disease are: prolonged antibiotic use (>2 months), advanced age (>65), hospitalization, and residency in a long-term care facility. Prolonged antibiotic exposure is considered the most significant risk factor at this time. Several recent studies have identified patients taking proton pump inhibitors (PPI) are much more susceptible to colonization than those who are not. Asseri et al found patients taking PPIs were 3.6 times more likely to acquire a C. Difficile infection than those who were not. Although, some researchers theorize that acid suppression is not responsible for increased infection rate, but is a marker of other co-morbidities that increase risk of C. Difficile infection (CDI).[4][5]
As noted earlier prolonged antibiotic use and advanced age are two significant risk factors for CDI. Though prolonged antibiotic use seems to be most significant for initial infection, patients with advanced aged are most susceptible for disease reoccurrence. This increased infection and reoccurrence rate in the elderly is likely due to ineffective immune responses as well as insufficient recovery of commensal microbiota following treatment with anti-CDI antibiotics. Recurrent CDI typically occurs shortly after cessation of anit-CDI antibiotic pharmaceuticals with a reappearance of symptoms within 14-45 days. Reoccurance following anti-biotic therapy is primarily contributed to persistent alteration in gut flora of the patient. The causative strain of the CDI reoccurrence is molecularly identical to the original strain in many patients. First time reoccurrence rates have been documented as 33% and infection following previous reoccurrence as high as 45%.[5][1][2]
A trend of increasing CDI rates was reported throughout the United States in the early 2000’s. Various studies aimed to identify the cause of this significant increase in infection rate and were able to identify particularly strains that were associated with higher rates of infection. Specifically, BI/NAP1/027 strain was identified as a strain primarily responsible for the increased infection rate. This particular strain exhibited an increased resistance to antibiotics used to treat CDI and produced more than twenties times more toxin than historical strains. This strain is also associated with infection of people not previously considered at risk including young, seemingly healthy individuals not exposed to a healthcare environment or prolonged antibiotic treatment. There is also an increased mortality rate in patients infected with BI/NAP1/027 when compared to traditional strains, especially in people 60-90 years old.[4][2][3]
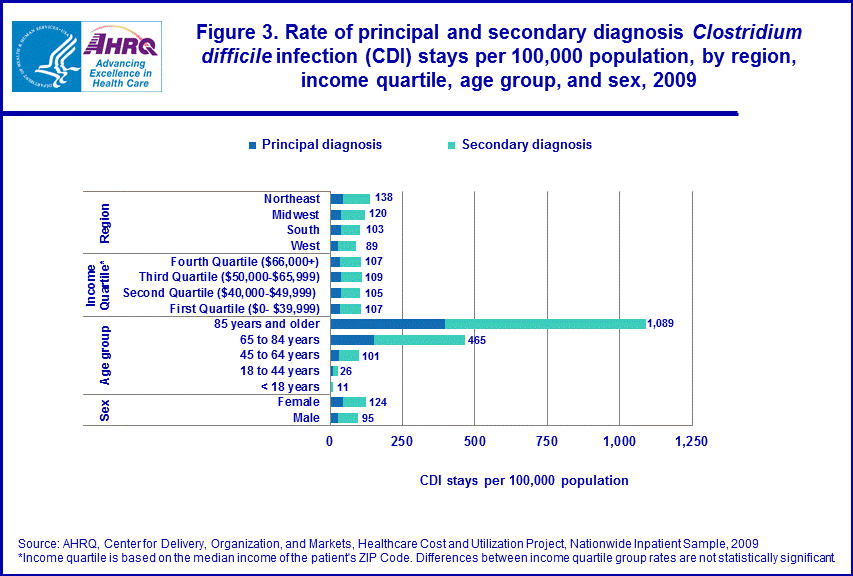
CDI Rates[6]
Characteristics/Clinical Presentation[edit | edit source]
Manifestations of CDI range from symptomless carriage, to mild-moderate diarrhea, to fatal pseudomembranous colitis. Fever, cramping, abdominal discomfort, and peripheral leukcytosis are common though found in less than half of patients with CDI. The passage of musucs, melena, or hematochezia are rare. Non-GI related symptoms such as arthritis and bacteremia have very rarely been reported in the literature. Patients who have undergone a complete colectomy have been reported to develop C. Difficile ileitis or puchitis in rare instances. Severe CDI can cause: dehydration, electrolyte disturbances, hypoalbuminemia, toxic megacolon, bowel perforation, hypotension, renal failure, sepsis, clonic ileus, toxic dilatation, systemic inflammatory response syndrome, and death. Patients presenting with unexplained leukocytosis should alarm the clinician and prompt them to request stool samples sent for diagnostic testing.[6][7][3][4][8]
Associated Co-morbidities[edit | edit source]
Co-morbidities such as cancer, old age, and renal disease were significantly correlated with a higher rate of mortality from CDI in an English Cohort study of over 2,000 people . Other significant risk factors mentioned in previous sections including: inflammatory bowel disease, immunocompromise, chemotherapy, abdominal surgery, gastrointestinal procedure, previous C. Difficile infection.[3]
Medications[2][9][edit | edit source]
- Vancomycin (Glycopeptide Antibiotics)
-Metroidazole (Nirtoimidazole Antibiotic)
- Rifamimin (Antimicrobial)
-Probiotics
-Immunosuppressants
Drug Characteristics[7] 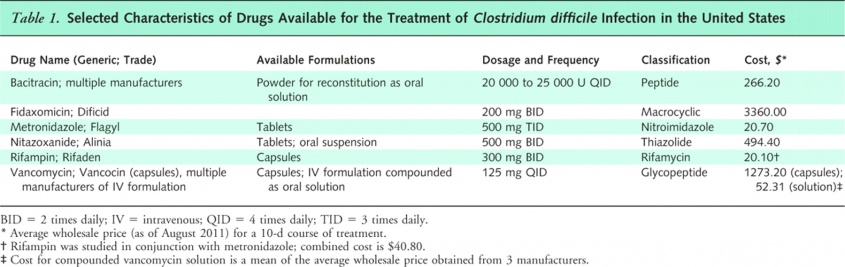
Diagnostic Tests/Lab Tests/Lab Values[edit | edit source]
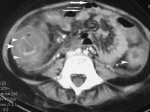
For definitive diagnosis a stool sample must be collected and tested for the antigen glutamate dehydrogenase (GDH). Testing for this antigen can be completed several different ways and is commonly done so via enzyme-linked immunisirbent assay (ELISA) or immunochromatographic assay. GD functions as cell-wall protein produced by the C. Difficile bacteria in considerably higher amounts than toxins. If GDH, TcdA, and TcdB are all present the physician can definitively diagnose the patient with CDI. There are a number of different tests used for identification of these markers, all of which have varying levels of psychometric properties. If all three markers are not present subsequent tests will be performed that are more specific to the missing markers before CDI is ruled out by the physician. Other tests occasionally used are sigmoidoscopy and colonoscopy. These tests are not frequently used because only ~50% of CDIs have visible pseudomembrane formation. Thickening of the colon wall and pericolonic stranding are sometimes evident on radiographs and referred to as “accordion sign” and or “double-halo sign.”[10][5]
Double Halo Sign[2]
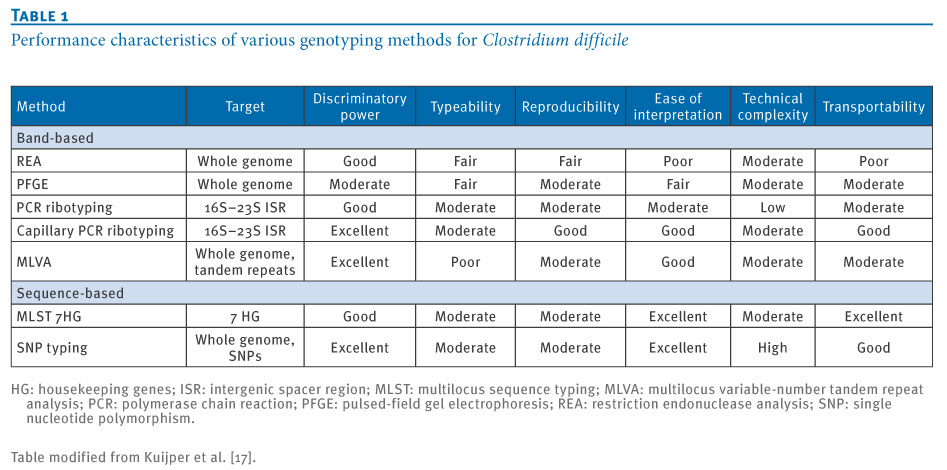
Genotyping Methods[5]
Etiology/Causes[edit | edit source]
C. Difficile bacteria colonize the large colon after exposure. Colonization in areas other than the large colon is quite rare in the human body. The natural anaerobic environment and absence of competing gut flora (due to antibiotic treatment) make the large colon an ideal environment for the bacteria to flourish.For the bacteria to flourish in the gut and damage the host several events must occur so that toxin production is possible. To start, the C. difficile spores must reach an area in the gut where an ample amount of bile salts are present so that they can catalyze the germination of these spores.[5][4][3]
After germination is complete, the bacteria begins produce toxins, initially focusing on a binary toxin that produces microtubule-cell extensions allowing adherence of the bacteria to the intestinal walls. During colonization and immediately following, C. difficile now in a vegetative form releases TcdA and TcdB. The mechanism of A and B toxin secretion is not fully understood, but the it is known that amion acid availability is critical to toxin expression. Primarily acting on the epithelial cells of the intestines, TcdA causes inflammation, fluid secretion, and necrosis of colon tissue. TcdB is a potent cytoxin that may be responsible for systemic damaged present in patients with fulminant CDI due to its cardiotoxic behavior.[5][4][3]
Systemic Involvement[3][edit | edit source]
-Dehydration
-Ectrolyte disturbances
-Hypoalbuminemia
-Toxic megacolon
-Bowel perforation
-Rrenal failure
-Sepsis
-Clonic ileus
-Toxic dilatation
-Systemic inflammatory response syndrome
-Death
Medical Management (current best evidence)[edit | edit source]
Medical management of CDI depends on the acuity and severity of the infection as well as whether it is an initial or recurrent infection. It is widely recognized that the most important first step in treatment is to discontinue the inciting antibiotic. Discontinuation of antibiotic treatment historically resulted in resolution of the infection, but due to new hypervirulent strains further intervention is recommended. Fluid and electrolyte replacement is suggested for quicker recovery. The available drugs for treatment of a CDI are Vancomycin and Metronidazole. If Vancomycin is the drug selected for treatment it should be administered either rectally or orally. These are the recommended routes of administration because intravenous Vancomycin has shown to be an ineffective treatment of CDI.[9]
On the other hand, Metronidazole has been shown to deliver similar colonic and intraluminal levels regardless of administration. Some researchers advocate oral administration when possible to avoid potential complications with an intravenous route. Though Metronidazole has been shown to be equally as effective as Vancomycin it has a higher rate of unresponsiveness. If patients do not respond to Metronidazole, Vancomycin is then considered. Vancomycin is more commonly the first treatment especially in severe cases of CDI due its lower rate of unresponsiveness and superior efficacy.[3][9]
Mild to moderate cases of CDI are treated with 25o mg of metronidazole every six hours or 125 mg of vacomycin every 6 hours. Treatment course is typically 10-14 days with resolution of symptoms in more than 90% of cases in less than 10 days. Combined use of these drugs is often used in patients with moderate to severe infection especially if accompanied by ileus and or toxic megacolon. Patients with unresolving ileus can be given increased doses as well as frequency of administration (i.e. 500 mg every 6 hours). In severe cases surgical evaluation for colectomy may be indicated to avoid life-threatening complications. In patients with reoccurrent infection pulse or tapering therapy is combined with vancomycin treatment. A course of Rifamimin is also recommended after previous treatments have failed.[9][2]
 Physical Therapy Management (current best evidence)[edit | edit source]
Physical Therapy Management (current best evidence)[edit | edit source]
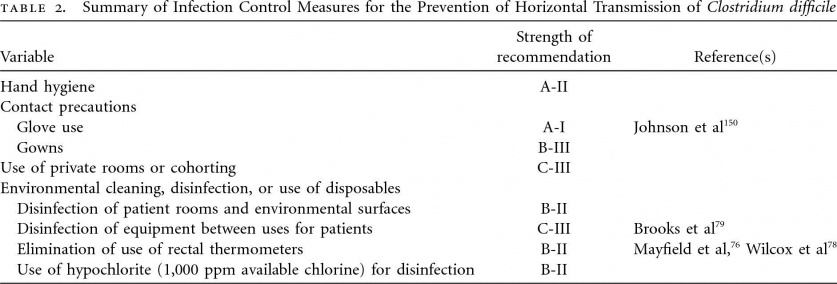
C. difficile infections are primarily managed through pharmaceutical therapy and associated medical treatments. Exercise recommendations for patients with CDI have not been clearly established and are currently based on clinical decision making of the therapeutic team and treating physician. The therapist should take an active role in infection control and prevention measures whenever working with these patients. Infection control measures include: donning gloves and gowns prior to entering patients room, washing hands with soap and water upon departure, treating patients in a private room, educating visitors on proper hygiene measures. Routine environmental screening and use of chlorine-containing cleaning agents is also recommended. Below is a table summarizing the evidence supporting various infection control measures.[4]
Infection Control[4]
Alternative/Holistic Management (current best evidence)[edit | edit source]
Though patients with CDI have a disturbance in native-gut flora, antimicrobial pharmaceuticals are being investigated as a potential treatment option. There are several antimicrobials under investigation including: tinidazole, rifaximin, rifalazil, nitazoxanide, glycopeptides derivatives, fidoxamicin, and topical bacitracin.[9]
Probiotics have also been proposed as a potential treatment option though evidence is not strong enough for it be considered as a primary option. Theoretically probiotics may replenish the native-flora of the colon which was depleted by prolonged anti-biotic treatment. This native flora would then compete with the C. difficile strains for nutrients and habitable space in the colon. Probiotics are reportedly non-pathogenic microorganisms though are not used in immunocompromised patients because of cases of blood-borne infections. The most widely studied probiotics are Saccharomyces and Lactobacillus. Moderate improvements have been demonstrated when probiotics are used in conjunction with vancomycin or metronidazol when compared to the ladder drugs alone. A single case report, demonstrated the effectiveness of reducing the incidence of CDI through concurrent treatment administration of probiotics and antimicrobials.[1]
Another developing treatment strategy is immunotherapy. Inconsistent results have been reported as to whether intravenous administration of human immunoglobulin can neutralize C. difficile toxins A and B in recurrent and sever CDIs. Recent research has reported when given in conjunction with vancomycin, human monoclonal antibodies can significantly reduce the reoccurance rate of CDI.
Lastly, research goal of practically every infectious disease is to develop a safe and effective vaccine. Research on a C. difficile vaccine is currently taking place and shown promising results in both animal and human volunteers. Since thorough analysis has not been performed on a large sample of patients its safety and efficacy to date is not established.[9]
Differential Diagnosis
[edit | edit source]
A more comprehensive list of potential causes of diarrhea and potential differential diagnoses include: pancreatitis, pancreatic carcinoma, Crohn’s disease, Irritable bowel syndrome, diabetic enteropathy, hyperthyroidism, caffeine, incomplete obstruction, fecal impaction, muscular incompetency, ileal bypass, viral infection, bacterial infection, parasitic infection, protozoal infection (Giardia), ulcerative colitis, diverticulitis, food allergy, creatine use, chemotherapy, lactose intolerance, psychogenic (nervous tension). A thorough assessment of patients history, signs, symptoms, risk factors, and lab tests are necessary for an accurate diagnosis.[3]
Case Reports/ Case Studies[edit | edit source]
A. Loshkajian. Pseudomembranous Colitis. Available at: http://www.eurorad.org/eurorad/case.php?id=1368. Accessed March, 17, 2014
Other Cases:
Pron B, Merckx J, Gaillard J, et al. Chronic septic arthritis and osteomyelitis in a prosthetic knee joint due to Clostridium difficile. European Journal Of Clinical Microbiology & Infectious Diseases: Official Publication Of The European Society Of Clinical Microbiology [serial online]. July 1995;14(7):599-601. Available from: MEDLINE, Ipswich, MA. Accessed March 12, 2014.
Bermejo C, Maseda E, Salgado P, Gabilondo G, Gilsanz F. [Septic shock due to a community acquired Clostridium difficile infection. A case study and a review of the literature.]. Revista Espanola De Anestesiologia Y Reanimacion[serial online]. June 1, 2013;Available from: MEDLINE, Ipswich, MA. Accessed March 12, 2014.
Leonard A, Ho K, Flexman J. Proton pump inhibitors and diarrhoea related to Clostridium difficile infection in hospitalised patients: a case-control study. Internal Medicine Journal [serial online]. May 2012;42(5):591-594. Available from: Academic Search Premier, Ipswich, MA. Accessed March 12, 2014.
De Almeida M, Heffernan H, Roberts S, et al. Severe Clostridium difficile infection in New Zealand associated with an emerging strain, PCR-ribotype 244. The New Zealand Medical Journal [serial online]. August 16, 2013;126(1380):9-14. Available from: MEDLINE, Ipswich, MA. Accessed March 12, 2014.
Resources
[edit | edit source]
http://www.cdc.gov/HAI/organisms/cdiff/Cdiff_infect.html
http://www.apic.org/Professional-Practice/CDIresources
http://www.in.gov/isdh/files/CDI_ResourceManual.pdf
References[edit | edit source]
see adding references tutorial.
Bellarmine_Student_Project
- ↑ 1.0 1.1 1.2 McFarland L. Meta-analysis of probiotics for the prevention of antibiotic associated diarrhea and the treatment of Clostridium difficile disease. The American Journal Of Gastroenterology [serial online]. April 2006;101(4):812-822. Available from: MEDLINE, Ipswich, MA. Accessed March 12, 2014
- ↑ 2.0 2.1 2.2 2.3 2.4 2.5 2.6 . A. Loshkajian. Pseudomembranous Colitis. Available at: http://www.eurorad.org/eurorad/case.php?id=1368. Accessed March, 17, 2014
- ↑ 3.00 3.01 3.02 3.03 3.04 3.05 3.06 3.07 3.08 3.09 3.10 Cecil J. Clostridium difficile: Changing Epidemiology, Treatment and Infection Prevention Measures. Current Infectious Disease Reports [serial online]. December 2012;14(6):612-619. Available from: MEDLINE, Ipswich, MA. Accessed March 17, 2014
- ↑ 4.0 4.1 4.2 4.3 4.4 4.5 4.6 4.7 4.8 Cohen S, Gerding D, Wilcox M, et al. Clinical practice guidelines for Clostridium difficile infection in adults: 2010 update by the Society for Healthcare Epidemiology of America (SHEA) and the Infectious Diseases Society of America (IDSA). Infection Control & Hospital Epidemiology [serial online]. May 2010;31(5):431-455. Available from: CINAHL, Ipswich, MA. Accessed March 12, 2014.
- ↑ 5.0 5.1 5.2 5.3 5.4 5.5 Knetsch C, Lawley T, Hensgens M, Corver J, Wilcox M, Kuijper E. Current application and future perspectives of molecular typing methods to study Clostridium difficile infections. Euro Surveillance: Bulletin Européen Sur Les Maladies Transmissibles = European Communicable Disease Bulletin [serial online]. January 24, 2013;18(4):20381. Available from: MEDLINE, Ipswich, MA. Accessed March 12, 2014
- ↑ 6.0 6.1 Lucado J, Gould C, Elixhauser A. Clostridium Difficile Infections (CDI) in Hospital Stays, 2009: Statistical Brief #124. [serial online]. February 2006;Available from: MEDLINE, Ipswich, MA. Accessed March 12, 2014
- ↑ 7.0 7.1 Garg S, Mirza Y, Dutta S, et al. Epidemiology of Clostridium difficile-Associated Disease (CDAD): A Shift from Hospital-Acquired Infection to Long-Term Care Facility-Based Infection. Digestive Diseases & Sciences [serial online]. December 2013;58(12):3407-3412. Available from: Academic Search Premier, Ipswich, MA. Accessed March 17, 2014
- ↑ Center for Disease Control and Prevention. Healthcare-Associated Infections. Available at: http://www.cdc.gov/hai/organisms/cdiff/Cdiff_clinicians.html. Accessed March 14, 2014.
- ↑ 9.0 9.1 9.2 9.3 9.4 9.5 Drekonja D, Butler M, Wilt T, et al. Comparative Effectiveness of Clostridium difficile Treatments: A Systematic Review. Annals Of Internal Medicine [serial online]. December 20, 2011;155(12):839-847. Available from: CINAHL, Ipswich, MA. Accessed March 14, 2014
- ↑ Heinlen L, Ballard J. 2010. Clostridium difficile infection. Am. J. Med. Sci.340:247–252. Accessed March 12, 2014