Cancer Cachexia
Definition[edit | edit source]
Cancer cachexia or cancer associated fatigue is defined as a multi factorial syndrome characterized by an ongoing loss of skeletal muscle mass (with or without loss of fat mass) that cannot be fully reversed by conventional nutritional support and leads to progressive functional impairment.[1]
The characteristic feature is the negative protein-energy balance that occurs due to reduction in the food intake and an abnormal metabolism.
Classification[edit | edit source]
There are 3 stages of relevance clinically, forming a spectrum, however, not all of these subjects traverse the entire spectrum.
- Precachecia: The early clinical signs like anorexia and metabolic signs like impaired glucose test precede weight loss (≤5%). The progression varies and depends on the cancer type and staging, low food intake, any systemic inflammation, poor response to anti cancer therapy.
- Cachexia: A stable weight loss more than 5% over 6 months OR a Body mass index (BMI) of lesser than 20 kg/m² OR sarcopenia and ongoing weight loss of more than 2%, but not yet entered the refractory stage classified as cachexia.
- Refractory cachexia: Very advanced cancer OR rapidly progressive cancer, unresponsive to anticancer therapy. Associated with active catabolism and the factors associated with active management of weight loss here no longer stand appropriate. Low performance status and a life expectancy of less than 3 months are characteristic.[2]
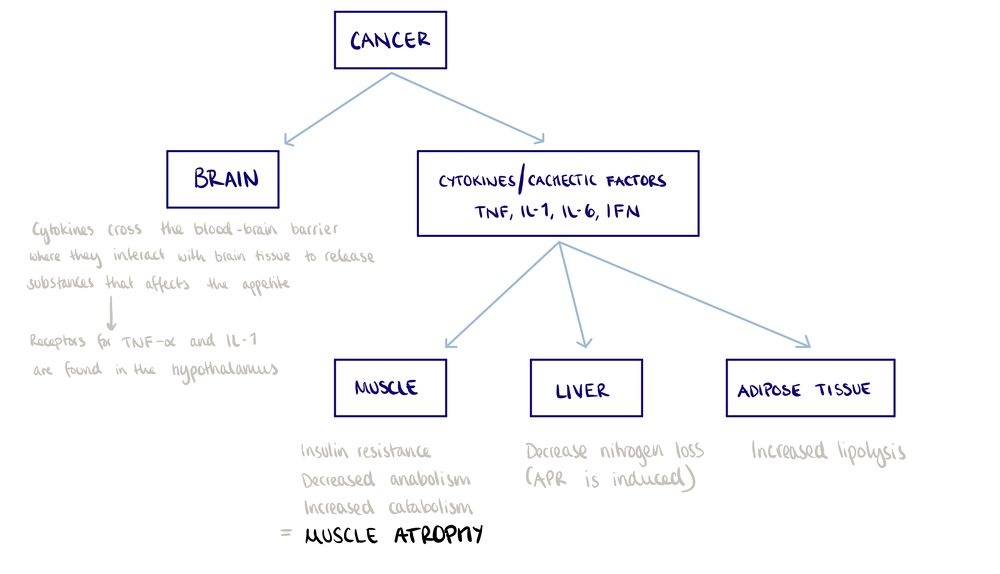
Pathophysiology[edit | edit source]
Diagnosis of Cancer Cachexia[edit | edit source]
- BMI <20 and any degree of weight loss >2%
- Weight loss >5% over past 6 months (in absence of simple starvation)
- Appendicular skeletal muscle index consistent with sarcopenia (males <7·26 kg/m²; females <5·45 kg/m²) and any degree of weight loss >2%[3]
Assessment[edit | edit source]
Muscle mass and strength[edit | edit source]
Cross sectional imaging CT or MRI, dual energy Xray imaging, bioimpedance analysis, and anthropometry mainly including the mid arm girth.
Anorexia and reduced food intake[edit | edit source]
Quantification of protein may be relevant. The mechanisms behind this may be chemosensory disturbances, reduced upper gastrointestinal mobility, distal tract dysmobility. The secondary causes include stomatitis, constipation, dyspnoea, pain and poor dietary habits.
Hypercatabolism[edit | edit source]
This could be due to tumor mediated side effects or systemic inflammation. Contributing factors include insulin resistance, long duration high dose corticosteroid, an elevation in the resting energy expenditure, hypogonadism.
Psychosocial and Functional aspects[edit | edit source]
Patient reported outcomes for physical functioning including European Organisation for Research and Treatment of Cancer, Quality of life Questionnaire, Patient completed Eastern Cooperative Oncology Group questionnaire.This can be followed by activity meters and checklists. The psycho social aspects could be identified by routine questions abut the persons psychological status.[1]
Management[edit | edit source]
There are 4 basic steps of treatment strategies:
- Correcting the cause of the impaired nutritional intake
- Adequate nutritional support
- Multimodal cancer cachexia intervention:
- Detecting any related psycho-social distress and treating the same[4]
Multimodal anabolic interventions are best in symptom management. Individualized nutrition and exercise optimize the drug effects[5] Counselling including behavioral change, anticancer or antineoplastic treatment, total parenteral nutrition, prokinetics, progestins, cannabinoids, Eicosapentaenoic acid, Cyclo-oxygenase inhibitors, Corticosteroids and exercise interventions are the treatments.[4]
Exercise Interventions[edit | edit source]
Mechanisms[edit | edit source]
Exercises can increase the muscle mass, muscle function, strength, cardiovascular fitness and reduce fatigue indirectly improving the quality of life by reducing fatigue levels. There are several suggested mechanisms for the same. They include[6]:
Exercise and inflammation[edit | edit source]
Acute exercise induce an immune response which increases the cytokine levels in the body, however, these cytokines no not produce the pro inflammatory effect. IL-6 is the typical cytokine released and expected in elevated levels, along with IL-10 and IL-1ra, This triggers an anti-inflammatory response which is speculated to reduce the systemic inflammation due to cancer, hence attenuating the cachexia process. Right from moderate intensity concentric exercises to vigorous intensity eccentric exercises, these interventions increase the rate of transcription an expresses the IL-6 protein which is necessary for the contracting muscle.
Inflammation and exercise in the adipose tissue[edit | edit source]
Endurance exercise block the effect of TNF-α, an inflammatory cytokine that stimulate lipolysis and support the inflammatory cascade.
Exercise and oxidative stress[edit | edit source]
Exercising enhances the antioxidative enzymes such as super-oxide dismutase, glutathione peroxidase in the skeletal muscle and mitochondrial superoxide dismutase and catalase are in the lungs and diaphragm. Also, the non-enzymatic antioxidant levels increase in the body thus protecting the tissues from damage.
Exercise and insulin sensitivity[edit | edit source]
It has been speculated that insulin resistance occurs in response to tumor growth and as the normal inflammatory response. Exercises reduces the TNF-α factor hence improving the body sensitivity to insulin. Also glucose transport proteins like Glutathione-4 increase in the skeletal muscles the increasing the glucose transport into the muscle. Creatinine phosphate that prevents the action of glutathione reduces while exercising.[6]
Exercises[edit | edit source]
- High-Intensity Interval training for 8 weeks has an impact on stage III and stage IV non-small cell lung cancer in patients who are receiving chemotherapy. These patients had a high risk of respiratory failure due to cancer cachexia and exercise training helped improve their lung capacity.
- Progressive resistance exercise training (2-3 days a week for 12 weeks) improved the patients' compliance and lean body mass by 1-2 Kgs. However, its effectiveness is yet to be determined in the head and neck cancer group receiving radiation therapy.
- Aerobic exercises are beneficial in terms that it increases the mitochondrial biogenesis and reduces the proteolysis by reducing the inflammation.[5]
- Pedometer based exercises(7 weeks) help in improving the skeletal mass, functional capacity, and quality of life in cancer cachexia on chemotherapy.[7]
- Resistance exercises in patients with prostrate cancer receiving androgen deprivation therapy and radiation therapy prevent loss of muscle mass and strength
References[edit | edit source]
- ↑ 1.0 1.1 Fearon K, Strasser F, Anker SD, Bosaeus I, Bruera E, Fainsinger RL, Jatoi A, Loprinzi C, MacDonald N, Mantovani G, Davis M. Definition and classification of cancer cachexia: an international consensus. Lancet Oncol. 2011 May 1;12(5):489-95.
- ↑ Blum D, Omlin A, Fearon K, Baracos V, Radbruch L, Kaasa S, Strasser F, European Palliative Care Research Collaborative. Evolving classification systems for cancer cachexia: ready for clinical practice?. Support Care Cancer. 2010 Mar 1;18(3):273-9.
- ↑ Wallengren O, Lundholm K, Bosaeus I. Diagnostic criteria of cancer cachexia: relation to quality of life, exercise capacity and survival in unselected palliative care patients. Support Care Cancer. 2013 Jun 1;21(6):1569-77.
- ↑ 4.0 4.1 Blum D, Omlin A, Fearon K, Baracos V, Radbruch L, Kaasa S, Strasser F, European Palliative Care Research Collaborative. Evolving classification systems for cancer cachexia: ready for clinical practice?. Support Care Cancer. 2010 Mar 1;18(3):273-9.
- ↑ 5.0 5.1 Anderson LJ, Albrecht ED, Garcia JM. Update on management of cancer-related cachexia. Curr Oncol Rep. 2017 Jan 1;19(1):3.
- ↑ 6.0 6.1 Gould DW, Lahart I, Carmichael AR, Koutedakis Y, Metsios GS. Cancer cachexia prevention via physical exercise: molecular mechanisms. J Cachexia Sarcopenia Muscle. 2013 Jun;4(2):111-24.
- ↑ Gandhi A, Samuel SR, Kumar KV, Saxena PU, Mithra P. Effect of a Pedometer-based Exercise Program on Cancer Related Fatigue and Quality of Life amongst Patients with Breast Cancer Receiving Chemotherapy. Asian Pac J Cancer Prev. 2020 Jun 1;21(6):1813-8.