Biomarkers of Parkinson's Disease
Original Editor - Arnold Fredrick D'Souza
Top Contributors - Arnold Fredrick D'Souza, Libby McConnell, Kim Jackson and Lucinda hampton
An introduction to Biomarkers[edit | edit source]
The Biomarkers Definitions Working Group defines biomarkers (biological markers) as ‘‘a measurable indicator of some biological state or condition that is objectively measured and evaluated to examine normal biological processes, pathogenic processes, or pharmacologic responses to a therapeutic intervention.’’ Biomarkers could be a used as a diagnostic tool, disease staging tool, prognostic tool, or for predicting and monitoring the clinical response to an intervention.[1] Biomarkers can have molecular, histological, radiographical, or physiological characteristics.[2]
Biomarkers and Parkinson's disease[edit | edit source]
Based on their characteristics, biomarkers of Parkinson's disease (PD) can be classified into three major categories[3][4][5]:
- Clinical
- Biochemical
- Neuroimaging
1. Clinical biomarkers[edit | edit source]
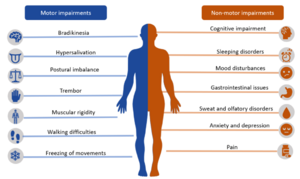
Motor features of PD[edit | edit source]
These include cardinal signs of PD which are resting tremors, bradykinesia or akinesia, rigidity (of lead-pipe or cogwheel variety), and postural instability.[3]Among them all, bradykinesia correlates the most with the degeneration of dopaminergic neurons in the nigrostriatal system.[5]
Non-motor features of PD[edit | edit source]
These include idiopathic rapid eye movement (REM) behavior disorder [characterized by the loss of atonia in REM sleep], hyposmia or anosmia (impaired very early during the course of the disease; also it is important to note that despite not being specific to PD, it is relatively uncommon in those with secondary Parkinsonism), and constipation (again being non-specific to PD in isolation, but its presence in combination with loss of olfaction and neuroimaging can allow for a more informed diagnosis of PD).[3]
All the above can be identified/quantified using clinical rating scales such as Hoehn and Yahr scale (H&Y), Unified Parkinson's Disease Rating Scale (UPDRS), Non-Motor Symptoms Scale for Parkinson's Disease (NMSS), and The Parkinson's Disease Questionnaire (PDQ-39) among many others.[3]
2. Biochemical biomarkers[edit | edit source]
Bodily fluids and tissue-based[edit | edit source]
Alpha-synuclein: It is a major component of Lewy bodies and is considered a pathological hallmark of PD.[3] It can be detected in blood, cerebrospinal fluid, saliva, urine, as well as the gastrointestinal tract.[5]
Oxidative stress: PD being a neurodegenerative disorder, signs of oxidative stress can be detected in the central nervous system. This can be detected by the presence of 8-hydroxydeoxyguanosine (8-OHdG), nitrotyrosine, and reactive oxygen species.[3]
Uric acid: It is a potential antioxidant or a free radical scavenger. In animal models, it has been found to prevent cell death in dopaminergic neurons. The levels of uric acid are significantly lower in the substantia nigra of individuals with PD.[3]
Genetic[edit | edit source]
Parkin (PARK2): It is commonly seen in those with juveline PD (ages under 20), an autosomal recessive variant of PD.[3]
Leucine-rich repeat kinase 2 (LRRK2): It is seen in the autosomal dominant variant of PD, which is most common among Caucasians, but it has also been detected in a relatively smaller section of Ashkenari Jews and North African Arabs.[3]
Glucocerebrosidase: It has been strongly associated with the onset of PD.[3]
3. Neuroimaging biomarkers[edit | edit source]
Radiotracer imaging: A scan of the nigrostriatal system can be used to detect signs of parkinsonism. Although, it is very expensive and non-specific to PD.[3]
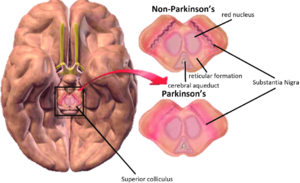
Transcranial ultrasound (TCUS): It can reveal a hyperechoic substantia nigra which is correlated with significantly higher iron content. It requires a great deal of technical expertise for this imaging technique to detect aforementioned changes and it is an expensive technique.[3]
Magnetic resonance imaging (MRI): It can show changes in diffusion tensor imaging (DTI) of the caudal section of the substantia nigra. This technique is very expensive as well.[3]
Application in physiotherapy research[edit | edit source]
Oral biomarkers of exercise-induced neuroplasticity in Parkinson's disease[edit | edit source]
Alpha-synucleiopathies or abnormal expressions of the alpha-synuclein protein, characteristically seen in the brain stem of persons with PD, can also be detected in saliva. The sympathetic and parasympathetic innervation of the sub-mandibulara glands are said to be responsible for this transduction via the central nervous system.[6]
Additionally, the following are some of the other oral biomarkers that have been found to increase in response to exercise[6]:
- Brain Derived Neurotrophic Factor (BDNF)
- Catalase (CAT)
- Dopamine Receptor D2 (DRD2)
- Glial Cell Line-Derived Neurotrophic Factor (GDNF)
- Glial Fibrillary Acidic Protein (GFAP)
- Glutamate Ionotropic Receptor AMPA type subunit 2 (GRIA2)
- Nitric Oxide Synthase 2 (NOS2)
- Super Oxide Dismutase (SOD1)
- Vascular Endothelial Growth Factor A (VEGFA)
Most of these findings are limited to animal studies with relatively limited research on humans.[6] This is a developing area of research and its findings will provide further evidence for the role of exercise in Parkinson's disease.
Wearable sensors as digital biomarkers of Parkinson's disease[edit | edit source]
Data collected from wearable sensors during the performance of the Timed Up and Go Test (TUG) has been studied to predict the frequency of falls in persons with Parkinson's disease. The authors used Quantitative Timed Up and Go (QTUG™) wherein a wireless inertial sensor is strapped to either leg. Further research is warranted for the validation of these results in a larger population, specifically in clinical settings.[7]
This video demonstrates the instrumentation and performance of Quantitative Timed Up and Go (QTUG™).[8]
References[edit | edit source]
- ↑ Biomarkers Definitions Working Group. Biomarkers and surrogate endpoints: preferred definitions and conceptual framework. Clin Pharmacol Ther. 2001 Mar;69(3):89-95.
- ↑ FDA-NIH Biomarker Working Group. BEST (Biomarkers, EndpointS, and other Tools) Resource [Internet]. Silver Spring (MD): Food and Drug Administration (US); 2016 Jan 28 [Updated 2021 Nov 29].
- ↑ 3.00 3.01 3.02 3.03 3.04 3.05 3.06 3.07 3.08 3.09 3.10 3.11 3.12 Morgan JC, Mehta SH, Sethi KD. Biomarkers in Parkinson's disease. Curr Neurol Neurosci Rep. 2010 Nov;10(6):423-30.
- ↑ Sharma S, Moon CS, Khogali A, Haidous A, Chabenne A, Ojo C, Jelebinkov M, Kurdi Y, Ebadi M. Biomarkers in Parkinson's disease (recent update). Neurochem Int. 2013 Sep;63(3):201-29.
- ↑ 5.0 5.1 5.2 Delenclos M, Jones DR, McLean PJ, Uitti RJ. Biomarkers in Parkinson's disease: Advances and strategies. Parkinsonism Relat Disord. 2016 Jan;22 Suppl 1(Suppl 1):S106-10.
- ↑ 6.0 6.1 6.2 Mougeot JL, Hirsch MA, Stevens CB, Mougeot F. Oral biomarkers in exercise-induced neuroplasticity in Parkinson's disease. Oral Dis. 2016 Nov;22(8):745-53.
- ↑ Greene BR, Premoli I, McManus K, McGrath D, Caulfield B. Predicting Fall Counts Using Wearable Sensors: A Novel Digital Biomarker for Parkinson’s Disease. Sensors. 2021 Dec 22;22(1):54.
- ↑ Kinesis QTUG - Quantitative Timed Up and Go - instructional video HD. Available from: https://www.youtube.com/watch?v=WqbEfhSBxlQ (accessed 23 May 2022)