Anterior Cruciate Ligament (ACL) - Structure and Biomechanical Properties
Original Editor - Tarang Jain
Top Contributors - Laura Ritchie, Kim Jackson, Admin, Mariam Hashem, Khloud Shreif, Tarang Jain, Evan Thomas, Jess Bell, 127.0.0.1, Tony Lowe, WikiSysop, Simisola Ajeyalemi, Olajumoke Ogunleye, Aminat Abolade, Robin Tacchetti, Ewa Jaraczewska and Rachael Lowe
Introduction[edit | edit source]
The Anterior Cruciate Ligament (ACL) is a key structure in the knee joint, as it resists anterior tibial translation and rotational loads. [1] It is one of the most frequently injured structures during high impact or sporting activities. [2] The ACL does not heal when torn, and surgical reconstruction is the standard treatment in the field of sports medicine. [3] Such reconstruction aims at restoring the kinematics and stability of the injured knee, to prevent future degenerative changes. [4] [5] Therefore, an adequate understanding of the complex anatomy, function, and biomechanics of the ACL is critical to elucidate the mechanisms of injury, understand the fate of chronic ACL deficiency, and to improve surgical reconstruction.
Development of the ACL[edit | edit source]
The knee originates from vascular femoral and tibial mesenchyme in the fourth week of gestation between the blastoma of femur and tibia. [6] [7] By 9 weeks, the Cruciate ligaments are composed of numerous immature fibroblasts having scanty cytoplasm and fusiform nuclei. [8] After week 20, the remaining development consists of marked growth with little change in form. At these stages two main bundles are already detectable, but the bundles seemed more parallel when compared to the bundle orientation of the adult ACL. [9] It is surrounded by a mesentery-like fold of synovium that originates from the posterior capsular apparatus of the knee joint. Thus, while the ACL is located intra-articularly, it remains extra-synovial throughout its course. [10]
The early manifestation of the ACL with two different bundles in the foetal knee suggests early development of the knee joint is guided by the ACL. Cruciate ligaments present at this early stage of development could lead to the assumption that they interact with the resulting shape of the femoral condyles and the tibial plateau. [5]
Gross Anatomy[edit | edit source]
Femoral Attachment[edit | edit source]
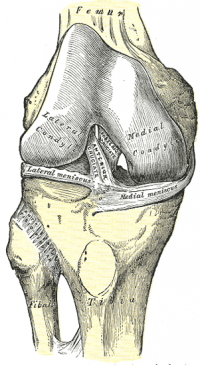
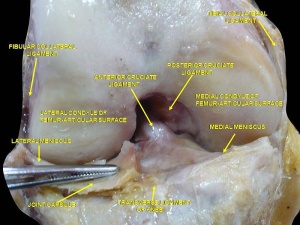
The ACL is a band like structure of dense connective tissues. The ACL is attached to a fossa on the posterior aspect of the medial surface of the lateral femoral condyle. [11] [7] The femoral attachment is in the form of a segment of a circle, with its anterior border straight and its posterior border convex. Its long axis is tilted slightly forward from the vertical, and the posterior convexity is parallel to the posterior articular margin of the lateral femoral condyle. [11] From its femoral attachment, the ACL runs anteriorly, medially, and distally to the tibia. Its length ranges from 22 to 41 mm (mean, 32 mm) and its width from 7 to 12 mm. [12]
Tibial Attachment[edit | edit source]
The ACL is attached to a fossa in front of and lateral to the anterior tibial spine. At this attachment the ACL passes beneath the transverse meniscal ligament, and a few fascicles of the ACL may blend with the anterior attachment of the lateral meniscus. In some instances, fascicles from the posterior aspect of the tibial attachment of the ACL may extend to, and blend with, the posterior attachment of the lateral meniscus. The tibial attachment of the ACL is somewhat wider and stronger than the femoral attachment. [11] The cross-sectional area increases from the femur to the tibia, as follows: 34 mm2 proximally, 33 mm2 mid-proximally, 35 mm2 at mid-substance level, 38 mm2 mid-distally, and 42 mm2 distally. [13] They also reported that the tibial insertion of the ACL is approximately 120% of the femoral insertion site. [14]
Spatial Orientation[edit | edit source]
The literature becomes confusing when the fascicular anatomy is categorised. Welsh (1980) and Arnoczky (1983) described the ACL as being the single broad continuum of fascicles, with different portions taut throughout the range of motion. [15] [16] However functionally, Girgis et al. divided the ACL into two parts, the anteromedial bundle (AMB) and the posterolateral bundle (PLB), [11] while other authors have separated the ACL in three functional bundles (AMB, intermediate band, and PLB). [12] [17]A recent study that utilised MRI deception and 3D visualisation observed three bundles in 22 knees (92%) and two bundles in 2 knees (8%) of the study subjects[18]. However, the two bundle model has been generally accepted as the best representation to understand ACL function.
The ACL courses anteriorly, medially, and distally across the joint as it passes from the femur to the tibia. As it does, it turns on itself in a slight outward (lateral) spiral. This is due to the orientation of its bony attachments. The orientation of the femoral attachment of the ACL, with regard to joint position (flexion/extension), is also responsible for the relative tension of the ligament throughout the range of motion. [7]
The ACL is attached to the femur and tibia, not as a singular cord, but rather as a collection of individual fascicles that fan out over a broad flattened area. [11] These fascicles have been summarily divided into two groups; the anteromedial band (AMB), those fascicles originating at the proximal aspect of the femoral attachment and inserting at the anteromedial aspect of the tibial attachment, and the posterolateral bulk (PLB), the remaining bulk of fascicles, which are inserted at the posterolateral aspect of the tibial attachment. In the frontal plane, the AMB has a more vertical orientation (approximately 70° to the knee base line) while the PLB is oriented more horizontally (approximately 55° to the knee base line). [7] When the knee is extended the PLB is tight, while the AMB is moderately lax. However, as the knee is flexed, the femoral attachment of the ACL assumes a more horizontal orientation, causing the AMB to tighten and the PLB to loosen and thus leave the AMB as the restraint to anterior tibial load. [11] Internal rotation lengthens the ACL a little more than does external rotation, most noticeably at 30° of flexion. Furthermore, Markolf et al. reported that ACL acts as a secondary restraint to varus-valgus angulation at full extension. [19] Twisting is resisted by a combination of capsular shearing, slanting collateral ligament action, joint surface, and meniscal geometry, while the cruciates play only a secondary role. [1]
While the two group designation provides a general idea as to the dynamics of the ACL through the range of motion, it oversimplifies somewhat. While a functional AMB is defined in flexion and a PLB is present in extension, the ACL is actually a continuum of fascicles, a different portion of which is taut throughout the range of motion. [16] This is of great clinical importance, because in any position of the knee, a portion of the ACL remains under tension and functional.
Recently, Zantop et al. suggested a classification of intra-articular rupture pattern of the ACL with regards to its two bundles. [20] This classification consists out of an alphanumeric code with letters for the location of the AM bundle rupture and numbers for the location of the PL bundle rupture. Femoral rupture location for the AM bundle is graded 1, mid-substance rupture is graded 2 and a tibial rupture of the AM bundle is graded 3. An elongated, functional insufficient AM bundle is graded 4 and an intact AM bundle 5. For the PL bundle, a rupture at the femoral origin, the mid-substance or the tibial insertion is graded A, B, and C, respectively. Elongated PL bundles are graded as D and intact PL bundle as E. The intra-operatively assessed rupture pattern of the AM and PL bundle can be expressed using this alphanumeric code; for example, 1A for a femoral rupture of the AM and a femoral rupture of the PL bundle. The validity and reliability of a possible classification is currently in development.
Micro Anatomy[edit | edit source]
The complex ultra-structural organisation, the varied orientation of the bundles in the ACL, and the abundant elastic system make it very different from other ligaments and tendons. The ACL is a unique and complex structure able to withstand multiaxial stresses and varying tensile strains. [21]
Microscopically, we can distinguish three zones within the ACL:
- The proximal part, which is less solid, is highly cellular, rich in round and ovoid cells, containing some fusiform fibroblasts, collagen type II and glycoproteins such as fibronectin and laminin.
- The middle part, containing fusiform and spindle shaped fibroblasts, is a high density of collagen fibres, a special zone of cartilage and fibrocartilage (especially in the anterior part where the ligament faces the anterior rim of the intercondylar notch), and elastic, and oxytalan fibres. The oxytalan fibres withstand modest multidirectional stresses, while elastic fibres absorb recurrent maximal stress. The fusiform and spindle-shaped fibroblasts are prominent in this middle part, which is also named the fusiform zone, and is located in the middle part and the proximal one-quarter of the ligament.
- The distal part, which is the most solid, is rich in chondroblasts and ovoid fibroblasts, and with a low density of collagen bundles. The fibroblasts, located on either side of the collagenous bundles are round to ovoid and resemble the cells of articular cartilage. In the anterior portion of the ACL, approximately 5–10 mm proximal to the tibial attachment, a layer of dense fibrous tissue surrounds the ligament instead of synovial tissue. This area corresponds to the zone where the ligament impinges on the anterior rim of the femoral intercondylar fossa in full knee extension.
The femoral origin and tibial insertion have the structure of a chondral apophyseal enthesis consisting of four layers. The first layer is composed of the ligament fibres. Fibro-cartilaginous cells aligned within the collagen bundles can be found in the second layer described as the non-mineralised cartilage zone, while the third layer is the mineralised cartilage zone. The fibrocartilage is mineralised and inserts into the subchondral bone plate, which is the fourth layer. [22] Due to this specific anatomy of the insertions, the ACL shows a transition zone from rigid bone to ligamentous tissue thereby allowing a graduated change in stiffness and may prevent stress concentration at the attachment site. [15] [23] [8]
The ligament itself consists of dense connective tissues and it is covered by synovial membrane. [24] The collagen fibrils are surrounded by connective tissue, which forms multiple fascicles in the ACL. [24] The major collagen of the ACL is Type I collagen, the loose connective tissue consists of Type III collagen. [25]
Interestingly, one anatomical study revealed differences in the structure of the anteromedial and posterolateral bundle. [25] In the anterior part of the anteromedial bundle, the typical cell morphology is different when compared with the typical structure of the rest of the ACL. In this region, the cells do not appear elongated. In full extension, this part of the ACL is in direct contact with the intercondylar fossa. [25] Histological sections of this area reveal typical tenocytes and chondrocyte-like cells. These chondroid cells even produce small amounts of the cartilage-specific Type II collagen. Because of the direct contact of the cartilage and the ligament, the appearance of chondrocytes could be explained as a functional adaptation of the ligament to compressive stress, which is caused by the physiological impingement between the ACL and the anterior rim of the intercondylar fossa. [25] Utilising quantitative polarised light imaging (QPLI) Skelley and colleagues [26] reported greater overall AM bundle stiffness and strength and more strongly aligned collagen fibres when loaded
Recently, Lee et al. found that oestrogen directly regulates ligament structure and function by alteration of type I and III synthesis. [27] [28] Indeed, oestrogen stimulates type I and III collagen synthesis at the mRNA level, while application of a mechanical force decreases the expression of collagen type I and III genes at all oestrogen levels tested. [27]
The parallel, dense, and regular organisation of ACL fibrils appears to be unique. It is a combination of helical and planar, parallel or twisted, nonlinear networks. The centrally located fascicles in the ACL are either straight or undulated in a planar wave pattern, whereas those located at the periphery are arranged in a helical wave pattern. The purpose of the wave and nonlinear pattern of the fibrils has been interpreted as "crimp" and "recruitment", respectively. [29] Crimp represents a regular sinusoidal pattern in the matrix. This accordion-like pattern in the matrix provides a "buffer" in which slight longitudinal elongation may occur without fibrous damage. It also provides a mechanism for control of tension and acts as a "shock-absorber" along the length of the tissue. [30] Hence, during tensile stretch, fibril ‘‘crimp’’ is first straightened out by small loads, after which larger loads are needed to elongate these fibrils. As such, an increasing number of fibrils become load bearing as larger loads are applied (‘‘recruitment’’) and a gradual increase in tissue stiffness is seen, resulting in a nonlinear load–elongation curve. This phenomenon allows the ACL to rapidly provide additional protection to the joint. [30]
Also recently, Chen et al. presented a human ACL model to evaluate the mechanical unloading effects on the histologic changes of ligament tissues over time. [31] Testing variables included fibroblast density, crimp amplitude, and crimp nuclear shape. The authors observed the sequential changes: Fibroblast density significantly increased within 5–6 weeks of unloading. By 7–8 weeks, crimp amplitude significantly decreased, accompanied by formation of irregular fibre patterns and fragments. This was followed by crimp wavelength and nuclear shape change from spindle to ovoid within 9–14 weeks. According to the literature, physical loadings provide an important stimulus for maintaining the normal structure and function of ligament tissue. Gene expression of type I and III collagen is also stimulated by mechanical stretch in ACL cells, via up-regulation of the transforming growth factor (TGF)-b1. [28] Therefore authors emphasised the important concept of early implementation of mechanical force in rehabilitation programs for patients with injured ligaments to prevent the deleterious effects due to mechanical unloading.
Biomechanics[edit | edit source]
The fibre bundles of the ACL do not function as a simple band of fibres with constant tension; in fact, they show a different tensioning pattern throughout a full range of motion. The differentiation of the ACL into two functional bundles, the anteromedial bundle (AMB) and posterolateral bundle (PLB), seems an oversimplification, but the two bundle description of the fibres of the ACL has widely been accepted as a basis for the understanding the function of the ACL. The terminology of the bundles was chosen according to their tibial insertion with the fibres of the AMB originating in the most proximal part of the femoral origin of the AMB and inserting at the anteromedial tibial insertion.[11] [23] [8] [11] As mentioned earlier, the role of AMB and PLB in restraining the anterior tibial translation is determined by their tensioning patterns throughout passive flexion–extension. Sakane et al. have shown that in response to 134 N anterior tibial load, the forces taken up by the PLB are higher in lower flexion degrees when compared to the AMB. [32] The AMB, however, was shown to take up more of the applied external force in higher flexion angles. [32] Using a liquid metal strain gage, Bach et al. reported higher strain in the PLB than in the AMB in knee flexion below 200. [3] A biomechanical study conducted on human cadavers reported insignificant increase in anterior tibial translation following partial tear of AM or PL bundles [33]. The reciprocal function between the two bundles remains inconclusive in the literature[34].
A recent study was performed using a robotic/universal force moment sensor and underlined the importance of the PLB. [35] In this study, the in situ forces of PLB in response to a 134 N anterior load were highest in full extension and decreased with increasing flexion. [35] These authors further demonstrated that the PLB plays a significant role in the stabilisation of the knee against a combined rotatory load. [35] A recent in vivo study using radiographic stereophotogrammetric analysis (RSA) evaluated the knee kinematics of ACL-reconstructed (single bundle technique) and uninjured (contralateral) knees of six subjects during downhill running. [36] The authors concluded that single bundle ACL reconstruction failed to restore normal rotational knee kinematics during dynamic loading. In conclusion, there seems to be some agreement favouring the hypothesis that the PLB is more of a restraint to tibial rotation than the AMB.
Following ACL rupture, rotational axis of the knee is altered compromising internal rotational instability[37]. As a result, movement at the postero-lateral component is increased by up to 413% at 15° of knee flexion[38].
Structural and Mechanical Properties[edit | edit source]
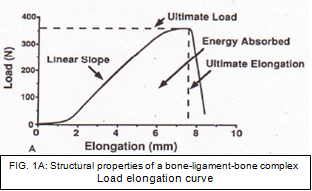
Structural properties can be described as the properties of the ligament or tendon together with its insertion site and fixation devices [39] while mechanical properties can be defined as the properties of the ligament or replacement graft itself without its insertion sites. [30] When a femur– ACL–tibia complex (FATC) is subjected to tensile testing, the resulting load-elongation curve represents the structural properties of the FATC (Fig. 1A). The shape of the curve depends on the properties of the ligament substance, the geometry of the complex, and the bone insertion site of the ligament. The important structural properties include the linear stiffness, ultimate load, ultimate deformation, and energy absorbed at failure (area beneath the curve). [40]
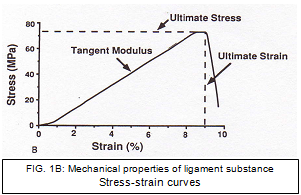
Although the structural properties provide valuable information about the FATC, they cannot tell us specifically about the material that composes the ligament. The “mechanical properties” of the ligament substance can be derived from the stress-strain curve (Fig. 1B). From the stress strain curve values for modulus, ultimate stress, and strain, energy density can also be determined. [39]
Structural and mechanical properties of the native ACL have been shown to decrease with higher age. [39] The mean ultimate load of FATC in specimens aged 22– 35 years was 2,160 (± 157) N. [39] The stiffness of an ACL reconstruction or the native FATC can be determined in load to failure tests as the linear region of the load elongation curve. For the specimens aged 22–35 years, the stiffness of the FATC was found to be 242 (± 28) N/mm. [39] The energy absorbed at failure can be calculated from the area beneath the curve and for specimens aged 22-35, the energy absorbed at failure was found to be 11.6 (± 1.7) Nm. [39]
The complex geometrical configuration and different-length fibre bundles of the ACL have hindered efforts to calculate stress and strain. Butler et al. divided the human ACL ligament into portions and tested the individual units for average modulus and ultimate tensile strength. [41] The average modulus and ultimate tensile strength measured 278 and 35 MPa, respectively. The ligaments reached their ultimate stress at -15% strain. In a later study Butler et al. found that AMB exhibited a larger modulus, ultimate tensile strength, and strain energy density than the posterior portion. [42]
The two most frequently used grafts are bone–patellar tendon–bone (BPTB) and hamstrings grafts as autologous tendon grafts. The goal of graft selection should be matching the load-elongation curve for ACL grafts to the curve generated by the human FATC. The structural properties of a 10 mm wide patellar tendon graft have been reported to be comparable with those of the native ACL with a mean ultimate failure strength of 1,784 (± 580) N and a mean stiffness of 210 N/mm. [43] The biomechanical analysis of a quadrupled hamstring graft revealed a mean ultimate load and stiffness of 2,422 (± 538) N and 238 N/mm, respectively. [43]However, these investigations were performed at time point zero and animal studies have shown that the structural properties of ACL reconstructions decrease due to healing and remodelling of the graft. Weiler et al. have shown in a sheep model that the tensile strength of a soft tissue graft fixed with interference screw fixation drops to 6.9% of that at time point zero and it might take up to 12 weeks until the strength level found at the time of reconstruction (time point zero) is reached again. [44]
Effects of muscle stabilisation[edit | edit source]
The muscles that cross the knee play a large role in maintaining the normal kinematics of the intact knee. Muscle activity can introduce large changes in the strains and forces experienced by the ACL. [7] Markolf et al. found that passive extension of the knee generated forces in the ACL only during the last 100° of extension, whereas a 200N quadriceps tendon forces in the ACL caused forces in the ACL to increase at all angles of knee flexion. [19] It has been demonstrated that quadriceps muscle forces induce increased anterior tibial translation whereas hamstring muscle forces have the opposite effect. With both quadriceps and hamstring forces, the strains of the AM portion are no different than in the unloaded knee throughout the angles of knee flexion. [45] Based on the force balance equations and geometric data from roentgenograms of healthy knees, Yasuda and Sasaki proposed that the quadriceps and hamstring muscles could be contacted simultaneously with the knee almost fully extended without producing a large anterior force. [46] Overall, studies suggest that passive flexion-extension motions, such as continuous passive motion, ranging from 100° of flexion to full flexion, are safe for rehabilitation of knee immediately after ACL reconstruction. Active flexion-extension motions should be limited to between 50° and 100°. Isometric quadriceps contraction should begin at or >70°. The quadriceps and hamstrings can be safely co-contracted at any flexion angles except full extension. [47]
Level walking, ascending and descending stairs were found to exert the highest shear forces on ACL compared to other activities such as sitting down, standing up and knee bending activities[48].
Recently, Zaffagnini et al. performed a qualitative and quantitative histological evaluation, by transmission electron microscopy (TEM), of the neoligamentisation process of a autologous bone-patellar tendon-bone (BTPB) graft used as pro-ACL at different follow-up times. [49] Their results showed that up to 24 months follow-up, progressive ultrastructural changes towards the normal ACL are observed. At longer times after surgery (48 and 120 months) no further changes were evident and the ultrastructure showed a marked reduction in large fibrils, which was typical of the control patellar tendon, and a significant increase in small fibrils. The ultrastructure seemed to combine fibrils from two different morphological units. The BPTB graft used as ACL underwent a transformation process for up to two years. After that period the transformation ceased and for ten years failed to reach the ultrastructural aspect of a normal ACL. However, from an architectural point of view the graft slowly transformed into a structure similar to ACL with respect to the different mechanical stresses the ligament has to sustain. [49] Similar study with autologous hamstring graft is in progress.
Also, Okahashi et al. recently evaluated whether the hamstring tendons can regrow after harvesting for anterior cruciate ligament (ACL) reconstruction and whether the regenerate tissue can be histologically characterised as tendinous. In their study, regeneration of the tendon was detected macroscopically in 9 of the 11 patients. Histologically and immunohistochemically, the regenerated tendons closely resembled normal ones. The results of this study show the hamstring tendons can regenerate after harvesting for the ACL reconstruction. [50] However, the use of hamstring grafts for ACL reconstruction can lead to different histological pattern of tendon-bone healing. Micromotion of the hamstring graft inside the drilled canal can be play a role in tendon-bone healing. [51]
A study that investigated the molecular characteristics of knee extensors following ACL-reconstruction found reduced slow fibre percentage, lowered mitochondria fibre density and capillary-to-fibre ratio particularly in vastus lateralis muscle up to 5 years after surgery[52].
References[edit | edit source]
- ↑ 1.0 1.1 Matsumoto, H., Suda, Y., Otani, T., Niki, Y., Seedhom, B. B., Fujikawa, K. (2001). Roles of the anterior cruciate ligament and the medial collateral ligament in preventing valgus instability. J Orthop Sci, 6(1), 28-32.
- ↑ Van Den Bogert, A. J., McLean, S. G. (2007). ACL injuries: do we know the mechanisms? J Orthop Sports Phys Ther, 37(2), A8-9.
- ↑ 3.0 3.1 Bach, B. R., Jr., Levy, M. E., Bojchuk, J., Tradonsky, S., Bush-Joseph, C. A., Khan, N. H. (1998). Single-incision endoscopic anterior cruciate ligament reconstruction using patellar tendon autograft. Minimum two-year follow-up evaluation. Am J Sports Med, 26(1), 30-40.
- ↑ Freedman, K. B., D'Amato, M. J., Nedeff, D. D., Kaz, A., Bach, B. R., Jr. (2003). Arthroscopic anterior cruciate ligament reconstruction: a metaanalysis comparing patellar tendon and hamstring tendon autografts. Am J Sports Med, 31(1), 2-11.
- ↑ 5.0 5.1 Lohmander, L. S., Ostenberg, A., Englund, M., Roos, H. (2004). High prevalence of knee osteoarthritis, pain, and functional limitations in female soccer players twelve years after anterior cruciate ligament injury. Arthritis Rheum, 50(10), 3145-3152.
- ↑ Petersen, W., Laprell, H. (2000). Insertion of autologous tendon grafts to the bone: a histological and immunohistochemical study of hamstring and patellar tendon grafts. Knee Surg Sports Traumatol Arthrosc, 8(1), 26-31.
- ↑ 7.0 7.1 7.2 7.3 7.4 Zantop, T., Petersen, W., Sekiya, J. K., Musahl, V., Fu, F. H. (2006). Anterior cruciate ligament anatomy and function relating to anatomical reconstruction. Knee Surg Sports Traumatol Arthrosc, 14(10), 982-992.
- ↑ 8.0 8.1 8.2 Petersen, W., Tillmann, B. (2002). [Anatomy and function of the anterior cruciate ligament]. Orthopade, 31(8), 710-718.
- ↑ Tena-Arregui, J., Barrio-Asensio, C., Viejo-Tirado, F., Puerta-Fonolla, J., Murillo-Gonzalez, J. (2003). Arthroscopic study of the knee joint in fetuses. Arthroscopy, 19(8), 862-868.
- ↑ Ellison, A. E., Berg, E. E. (1985). Embryology, anatomy, and function of the anterior cruciate ligament. Orthop Clin North Am, 16(1), 3-14.
- ↑ 11.0 11.1 11.2 11.3 11.4 11.5 11.6 11.7 Girgis, F. G., Marshall, J. L., Monajem, A. (1975). The cruciate ligaments of the knee joint. Anatomical, functional and experimental analysis. Clin Orthop Relat Res(106), 216-231.
- ↑ 12.0 12.1 Amis, A. A., Dawkins, G. P. (1991). Functional anatomy of the anterior cruciate ligament. Fibre bundle actions related to ligament replacements and injuries. J Bone Joint Surg Br, 73(2), 260-267.
- ↑ Harner, C. D., Livesay, G. A., Kashiwaguchi, S., Fujie, H., Choi, N. Y., Woo, S. L. (1995). Comparative study of the size and shape of human anterior and posterior cruciate ligaments. J Orthop Res, 13(3), 429-434.
- ↑ Harner, C. D., Baek, G. H., Vogrin, T. M., Carlin, G. J., Kashiwaguchi, S., Woo, S. L. (1999). Quantitative analysis of human cruciate ligament insertions. Arthroscopy, 15(7), 741-749.
- ↑ 15.0 15.1 Arnoczky, S. P. (1983). Anatomy of the anterior cruciate ligament. Clin Orthop Relat Res(172), 19-25.
- ↑ 16.0 16.1 Welsh, R. P. (1980). Knee joint structure and function. Clin Orthop Relat Res(147), 7-14.
- ↑ Hollis, J. M., Takai, S., Adams, D. J., Horibe, S., Woo, S. L. (1991). The effects of knee motion and external loading on the length of the anterior cruciate ligament (ACL): a kinematic study. J Biomech Eng, 113(2), 208-214.
- ↑ Otsubo H, Akatsuka Y, Takashima H, Suzuki T, Suzuki D, Kamiya T, Ikeda Y, Matsumura T, Yamashita T, Shino K. MRI depiction and 3D visualization of three anterior cruciate ligament bundles. Clinical Anatomy. 2017 Mar;30(2):276-83.
- ↑ 19.0 19.1 Markolf, K. L., Mensch, J. S., Amstutz, H. C. (1976). Stiffness and laxity of the knee--the contributions of the supporting structures. A quantitative in vitro study. J Bone Joint Surg Am, 58(5), 583-594.
- ↑ Zantop, T., Brucker, P. U., Vidal, A., Zelle, B. A., Fu, F. H. (2007). Intraarticular rupture pattern of the ACL. Clin Orthop Relat Res, 454, 48-53.
- ↑ Zaffagnini, S., Golano, P., Farinas, O., Depasquale, V., Strocchi, R., Cortecchia, S., et al. (2003). Vascularity and neuroreceptors of the pes anserinus: anatomic study. Clin Anat, 16(1), 19-24.
- ↑ Fu, F. H., Bennett, C. H., Lattermann, C., Ma, C. B. (1999). Current trends in anterior cruciate ligament reconstruction. Part 1: Biology and biomechanics of reconstruction. Am J Sports Med, 27(6), 821-830.
- ↑ 23.0 23.1 Dienst, M., Burks, R. T., Greis, P. E. (2002). Anatomy and biomechanics of the anterior cruciate ligament. Orthop Clin North Am, 33(4), 605-620.
- ↑ 24.0 24.1 Pitaru, S., Aubin, J. E., Bhargava, U., Melcher, A. H. (1987). Immunoelectron microscopic studies on the distributions of fibronectin and actin in a cellular dense connective tissue: the periodontal ligament of the rat. J Periodontal Res, 22(1), 64-74.
- ↑ 25.0 25.1 25.2 25.3 Petersen, W., Tillmann, B. (1999). Structure and vascularization of the cruciate ligaments of the human knee joint. Anat Embryol (Berl), 200(3), 325-334.
- ↑ Skelley NW, Lake SP, Brophy RH. Microstructural properties of the anterior cruciate ligament. Annals of Joint. 2017 May 23;2(5).
- ↑ 27.0 27.1 Kim, S. G., Akaike, T., Sasagaw, T., Atomi, Y., Kurosawa, H. (2002). Gene expression of type I and type III collagen by mechanical stretch in anterior cruciate ligament cells. Cell Struct Funct, 27(3), 139-144.
- ↑ 28.0 28.1 Lee, C. Y., Smith, C. L., Zhang, X., Hsu, H. C., Wang, D. Y., Luo, Z. P. (2004). Tensile forces attenuate estrogen-stimulated collagen synthesis in the ACL. Biochem Biophys Res Commun, 317(4), 1221-1225.
- ↑ Smith, B. A., Livesay, G. A., Woo, S. L. (1993). Biology and biomechanics of the anterior cruciate ligament. Clin Sports Med, 12(4), 637-670.
- ↑ 30.0 30.1 30.2 Woo, S. L., Gomez, M. A., Seguchi, Y., Endo, C. M., Akeson, W. H. (1983). Measurement of mechanical properties of ligament substance from a bone-ligament-bone preparation. J Orthop Res, 1(1), 22-29.
- ↑ Chen, C. H., Liu, X., Yeh, M. L., Huang, M. H., Zhai, Q., Lowe, W. R., et al. (2007). Pathological changes of human ligament after complete mechanical unloading. Am J Phys Med Rehabil, 86(4), 282-289.
- ↑ 32.0 32.1 Sakane, M., Fox, R. J., Woo, S. L., Livesay, G. A., Li, G., & Fu, F. H. (1997). In situ forces in the anterior cruciate ligament and its bundles in response to anterior tibial loads. J Orthop Res, 15(2), 285-293.
- ↑ Kondo E, Merican AM, Yasuda K, Amis AA. Biomechanical analysis of knee laxity with isolated anteromedial or posterolateral bundle–deficient anterior cruciate ligament. Arthroscopy: The Journal of Arthroscopic & Related Surgery. 2014 Mar 1;30(3):335-43.
- ↑ Domnick C, Raschke MJ, Herbort M. Biomechanics of the anterior cruciate ligament: Physiology, rupture and reconstruction techniques. World journal of orthopedics. 2016 Feb 18;7(2):82.
- ↑ 35.0 35.1 35.2 Gabriel, M. T., Wong, E. K., Woo, S. L., Yagi, M., Debski, R. E. (2004). Distribution of in situ forces in the anterior cruciate ligament in response to rotatory loads. J Orthop Res, 22(1), 85-89.
- ↑ Tashman, S., Collon, D., Anderson, K., Kolowich, P., Anderst, W. (2004). Abnormal rotational knee motion during running after anterior cruciate ligament reconstruction. Am J Sports Med, 32(4), 975-983.
- ↑ Amis AA, Bull AM, Lie DT. Biomechanics of rotational instability and anatomic anterior cruciate ligament reconstruction. Operative Techniques in Orthopaedics. 2005 Jan 1;15(1):29-35.
- ↑ Kanamori A, Sakane M, Zeminski J, Rudy TW, Woo SL. In-situ force in the medial and lateral structures of intact and ACL-deficient knees. Journal of orthopaedic science. 2000 Nov 1;5(6):567-71.
- ↑ 39.0 39.1 39.2 39.3 39.4 39.5 Woo, S. L., Hollis, J. M., Adams, D. J., Lyon, R. M., Takai, S. (1991). Tensile properties of the human femur-anterior cruciate ligament-tibia complex. The effects of specimen age and orientation. Am J Sports Med, 19(3), 217-225.
- ↑ Takeda, Y., Xerogeanes, J. W., Livesay, G. A., Fu, F. H., Woo, S. L. (1994). Biomechanical function of the human anterior cruciate ligament. Arthroscopy, 10(2), 140-147.
- ↑ Butler, D. L., Kay, M. D., Stouffer, D. C. (1986). Comparison of material properties in fascicle-bone units from human patellar tendon and knee ligaments. J Biomech, 19(6), 425-432.
- ↑ Butler, D. L., Guan, Y., Kay, M. D., Cummings, J. F., Feder, S. M., Levy, M. S. (1992). Location-dependent variations in the material properties of the anterior cruciate ligament. J Biomech, 25(5), 511-518.
- ↑ 43.0 43.1 Wilson, T. W., Zafuta, M. P., Zobitz, M. (1999). A biomechanical analysis of matched bone-patellar tendon-bone and double-looped semitendinosus and gracilis tendon grafts. Am J Sports Med, 27(2), 202-207.
- ↑ Weiler, A., Hoffmann, R. F., Bail, H. J., Rehm, O., Sudkamp, N. P. (2002). Tendon healing in a bone tunnel. Part II: Histologic analysis after biodegradable interference fit fixation in a model of anterior cruciate ligament reconstruction in sheep. Arthroscopy, 18(2), 124-135.
- ↑ Draganich, L. F., Vahey, J. W. (1990). An in vitro study of anterior cruciate ligament strain induced by quadriceps and hamstrings forces. J Orthop Res, 8(1), 57-63.
- ↑ Yasuda, K., Sasaki, T. (1987). Exercise after anterior cruciate ligament reconstruction. The force exerted on the tibia by the separate isometric contractions of the quadriceps or the hamstrings. Clin Orthop Relat Res(220), 275-283.
- ↑ Dubljanin-Raspopovic, E., Kadija, M., Matanovic, D. (2005). [Basic principles of aggressive rehabilitation after anterior cruciate ligament reconstruction]. Srp Arh Celok Lek, 133(11-12), 528-531.
- ↑ Marieswaran M, Jain I, Garg B, Sharma V, Kalyanasundaram D. A Review on Biomechanics of Anterior Cruciate Ligament and Materials for Reconstruction. Applied bionics and biomechanics. 2018;2018.
- ↑ 49.0 49.1 Zaffagnini, S., De Pasquale, V., Marchesini Reggiani, L., Russo, A., Agati, P., Bacchelli, B., et al. (2007). Neoligamentization process of BTPB used for ACL graft: histological evaluation from 6 months to 10 years. Knee, 14(2), 87-93.
- ↑ Okahashi, K., Sugimoto, K., Iwai, M., Oshima, M., Samma, M., Fujisawa, Y., et al. (2006). Regeneration of the hamstring tendons after harvesting for arthroscopic anterior cruciate ligament reconstruction: a histological study in 11 patients. Knee Surg Sports Traumatol Arthrosc, 14(6), 542-545.
- ↑ Nebelung, W., Becker, R., Urbach, D., Ropke, M., Roessner, A. (2003). Histological findings of tendon-bone healing following anterior cruciate ligament reconstruction with hamstring grafts. Arch Orthop Trauma Surg, 123(4), 158-163.
- ↑ Flück M, Viecelli C, Bapst AM, Kasper S, Valdivieso P, Franchi MV, Ruoss S, Lüthi JM, Bühler M, Claassen H, Hoppeler H. Knee extensors muscle plasticity over a 5-years rehabilitation process after open knee surgery. Frontiers in physiology. 2018;9.