Amyloidosis
Original Editor - Lucinda hampton
Top Contributors - Lucinda hampton and Uchechukwu Chukwuemeka
Introduction[edit | edit source]
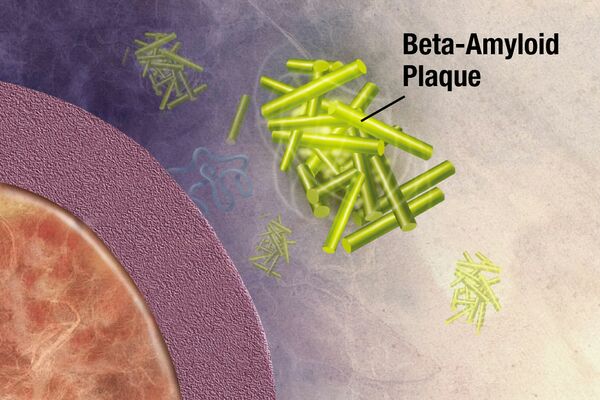
Amyloidosis is a heterogeneous disease that results from the deposition of toxic insoluble beta-sheet fibrillar protein aggregates in different tissues, resulting in disruption and impairment of organ structure and function. Amyloidosis affects multiple body organs and systems.[1][2][3]
Amyloidosis can be acquired or hereditary. The disease can be localized or systemic. Amyloid can accumulate in the liver, spleen, kidney, heart, nerves, and blood vessels, causing different clinical syndromes.[3]
Although awareness about amyloidosis has improved amongst the medical profession over the last decade many patients still experience symptoms and visit numerous specialists (often for months) before receiving a correct diagnosis.[1]
Amyloid[edit | edit source]
Amyloid is an amorphous, extracellular, insoluble fibrous proteins deposited in various body tissues and organs, giving rise the disease known as amyloidosis. One of its' characteristics is a β-sheet-rich secondary structure. The word “amylon” was first used in 1834, to describe the waxy starch in plants. In 1854 the word “amyloid” was coined to describe tissue deposits that stained like cellulose when exposed to iodine.[3]
- Amyloid formation involves a protein misfolding reaction. Amyloid fibrils are generally very stable and quite insoluble. Amyloid is a foreign substance composed of self-proteins, however the immune system does not easily recognise it nor remove it as a foreign substance. The reasons for this are not well understood.[4]
- Amyloid may be present as microscopic deposits, as plaques, or as masses that may grow to eventually replace the functional tissue of affected organs. Progressive loss of function, eventual organ failure and death may follow.[5]
- Twenty-one different proteins have been identified as amyloidogenic agents. Polypeptides can adopt alternative misfolded states, making them prone to aggregation. There are multiple processes by which misfolding of protein precursors occur. [2][1]
Types[edit | edit source]
- Primary amyloidosis: associated with monoclonal plasma cell dyscrasias
- Secondary amyloidosis: usually occurs secondary to a tissue destructive and inflammatory process: TB , Rheumatoid Arthritis , Multiple Myeloma , Crohn Disease , Ankylosing Spondylitis, Sjogren Syndrome, Dermatomyositis
- Hereditary amyloidosis: can be seen with familial Mediterranean fever (FMF)
- Senile amyloidosis
- Localised amyloidosis: sometimes classified as a separate entity with the above four accounting for the systemic forms
- Dialysis-related amyloidosis 7: can occur with either haemodialysis or peritoneal dialysis[3]
Neurodegenerative Disease[edit | edit source]
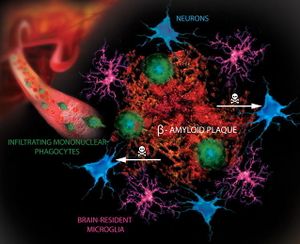
The protein abnormalities of almost all common neurodegenerative diseases have some characteristics of amyloid. In neurodegenerative diseases, amyloid-like filamentous aggregates are mostly within the cytoplasm of neurons and glia. Extracellular deposits of amyloid can be found in the brain parenchyma as plaques or in the walls of blood vessels as amyloid angiopathy. eg Gerstmann–Sträussler–Scheinker disease, Creutzfeldt–Jakob disease, and Alzheimer’s disease are all Amyloidoses[6]
Alzheimer’s Disease.[edit | edit source]
A lot of evidence supports the mechanism by which amyloid accumulation in the brain increases the risk of Alzheimer’s disease.
- Amyloid accumulation is caused by many factors, for example: impairment of cellular autophagy; low cerebral blood flow.
- Alternatively, studies on Alzheimer’s disease have shown that oxidative damage, tau protein hyperphosphorylation, and neurofibrillary tangles are among the etiologies of amyloid-related AD.
In recent years, drugs targeting the accumulation of amyloid have shown potential for future treatment, and beta amyloid is an important drug target for the treatment of AD.[7]
References[edit | edit source]
- ↑ 1.0 1.1 1.2 A.A.N What is Amyloidosis? Available:https://aan.org.au/health-professionals/what-is-amyloidosis/ (accessed 18.9.2022)
- ↑ 2.0 2.1 Bustamante JG, Zaidi SR. Continuing Education Activity.Available:https://www.ncbi.nlm.nih.gov/books/NBK470285/ (accessed 18.9.20220
- ↑ 3.0 3.1 3.2 3.3 Radiopedia Amyloidosis Available:https://radiopaedia.org/articles/amyloidosis (accessed 18.9.2022)
- ↑ Lennarz WJ, Lane MD. Encyclopedia of biological chemistry. Academic Press; 2013 Jan 8.Available: https://www.sciencedirect.com/topics/medicine-and-dentistry/amyloid (accessed 18.9.2022)
- ↑ Bancroft JD, Gamble M, editors. Theory and practice of histological techniques. Elsevier health sciences; 2008. Available: https://www.sciencedirect.com/topics/medicine-and-dentistry/amyloid (accessed 18.9.2022)
- ↑ Neurodegenerative Disease
- ↑ Ma C, Hong F, Yang S. Amyloidosis in Alzheimer’s disease: Pathogeny, etiology, and related therapeutic directions. Molecules. 2022 Feb 11;27(4):1210.Available: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC8876037/(accessed 18.9.20220