Preeclampsia
Original Editor - Lucinda hampton
Top Contributors - Lucinda hampton, Ayodeji Mark-Adewunmi and Kirenga Bamurange Liliane
Introduction[edit | edit source]
Pre-eclampsia is a pregnancy-specific multi-system disorder, marked by high blood pressure and often a significant presence of protein in the urine.[1] It typically starts after 20 weeks of pregnancy. Severe cases may lead to red blood cell breakdown, a low blood platelet count, impaired liver function, kidney dysfunction, swelling, shortness of breath from fluid in the lungs, or visual disturbances. Pre-eclampsia can lead to harmful outcomes for both mother and fetus, including preterm labor.[2] Untreated, it can escalate to seizures, a condition known as eclampsia.
Risk factors for pre-eclampsia include obesity, prior hypertension, advanced age, and diabetes mellitus.[3] It is more common in a woman's first pregnancy or when carrying twins. The condition involves complex mechanisms, including abnormal blood vessel formation in the placenta. Most cases are diagnosed before delivery and can be categorized by the gestational week. Pre-eclampsia often continues into the post-delivery period, known as postpartum pre-eclampsia.[4] Occasionally, it may start after delivery. Traditionally, diagnosis required high blood pressure and protein in the urine, but some definitions now include hypertension with any organ dysfunction.
High blood pressure is defined as greater than 140 mmHg systolic or 90 mmHg diastolic on two separate occasions, more than four hours apart, in a woman after twenty weeks of pregnancy. Pre-eclampsia is routinely screened for during prenatal care.
Preeclampsia is one of the leading causes of maternal and perinatal morbidity and mortality, causing difficulties in 2-8% of all pregnancies worldwide. Left untreated, women with preeclampsia are at lasrge risk risk for seizures (eclampsia), pulmonary edema, stroke, liver and kidney failure, and death. [5]
- Preeclampsia is a life-threatening cardiovascular disorder associated with pregnancy. Preeclampsia is marked by hypertension and proteinuria at 20 weeks of gestation. The underlying cause is not precisely known but likely heterogenous. Ample research suggests that for some women with preeclampsia, both maternal and placental vascular impairment plays a role in its' evolution and may carry on into the postpartum period.
- Preeclampsia is one of three conditions that constitute the syndrome of ischaemic placental disease, a group of pathologies that also includes placental abruption and intrauterine growth restriction.[6].[7]
Note: Hypertensive disorders of pregnancy are a leading cause of maternal and neonatal morbidity and mortality. Hypertension in pregnancy should be defined as a hospital systolic blood pressure (SBP) ⩾ 140 mmHg and/or diastolic blood pressure (DBP) ⩾ 90 mmHg, based on the average of at least two measurements, taken at least 15 min apart, using the same arm. To see Physiopedia's comprehensive page on Hypertensive disorders of pregnancy see here
Epidemiology[edit | edit source]
Preeclampsia and eclampsia contribute to over 50,000 maternal deaths annually worldwide. The incidence of preeclampsia is linked to ethnicity and race, with the highest prevalence among African-American and Hispanic individuals, accounting for approximately 26% of maternal deaths in these groups.[8]
Numerous risk factors and predeterminants for preeclampsia exist, including having no previous births, pregnancies with multiple gestations, maternal age over 35, use of in-vitro fertilization or other assisted reproductive technologies, and maternal comorbidities such as chronic hypertension, kidney disease, diabetes, thrombophilia, obstructive sleep apnea, and obesity with a pre-pregnancy BMI over 30. A family history of the condition, previous experiences of placental abruption or preeclampsia, and intrauterine fetal growth restriction also contribute to the risk.
Etiology[edit | edit source]
The exact cause of pre-eclampsia is not definitively known, but it is believed to be linked to various factors. These include abnormal placentation (formation and development of the placenta), immunologic factors, and existing maternal conditions such as hypertension, obesity, or antiphospholipid antibody syndrome, especially in those with a history of pre-eclampsia. Dietary factors, like low calcium intake, and environmental factors, such as air pollution, also play a role. Individuals with long-term high blood pressure are 7 to 8 times more at risk.[9]
Physiologically, pre-eclampsia has been associated with changes such as altered interactions between the maternal immune system and the placenta, placental and endothelial cell injury, altered vascular reactivity, oxidative stress, an imbalance of vasoactive substances, reduced intravascular volume, and disseminated intravascular coagulation.
Evidence suggests that a major predisposing factor for pre-eclampsia in susceptible women is an abnormally implanted placenta, leading to poor uterine and placental perfusion, hypoxia, increased oxidative stress, and the release of anti-angiogenic proteins and inflammatory mediators into the maternal bloodstream. This can result in widespread endothelial dysfunction, which causes hypertension and other symptoms and complications of pre-eclampsia. The abnormal implantation is thought to arise from the maternal immune system's response to the placenta, particularly a lack of established immunological tolerance in pregnancy. Endothelial dysfunction is a key factor in the development of hypertension and the various other symptoms and complications linked to pre-eclampsia.
Risk Factors[edit | edit source]
Recognized risk factors for pre-eclampsia include:
- Never having given birth
- Diabetes mellitus[10]
- Endometriosis
- Obesity
- Advanced maternal age (over 35 years)
- Kidney disease
- Untreated hypertension
- Previous history of pre-eclampsia
- Family history of pre-eclampsia
- Antiphospholipid antibody syndrome
- Multiple gestation[11]
- Having donated a kidney
- Sub-clinical hypothyroidism or thyroid antibodies[12][13]
- Placental abnormalities, such as placental ischemia
Socioeconomic factors significantly influence the prevalence of these risk factors and, as with other conditions, each factor contributes to the likelihood of increased morbidity and the complexity of care required for hospitalized patients.
Pathogenesis[edit | edit source]
Despite extensive research into the mechanisms of pre-eclampsia, its precise pathogenesis remains elusive. Pre-eclampsia is believed to originate from an abnormal placenta, whose removal typically resolves the condition. Normally, the placenta develops vasculature to facilitate the exchange of water, gases, and solutes, including nutrients and waste, between the mother and fetus. However, abnormal placental development can lead to inadequate placental perfusion. In pre-eclampsia, the placenta is often marked by insufficient trophoblastic invasion, which is thought to cause oxidative stress, hypoxia, and the release of factors that induce endothelial dysfunction, inflammation, and other potential responses.[14]
During the early stages of embryonic development, the outer epithelial layer is composed of cytotrophoblast cells. These stem cells, located in the trophoblast, eventually differentiate into the fetal placenta. They give rise to various placental cell types, including the extravillous trophoblast cells. These particular cells are invasive and play a crucial role in remodeling the maternal spiral arteries. They do this by replacing the maternal epithelial and smooth muscle cells lining the arteries, which leads to and sustains the dilation of the spiral arteries. This process is vital as it prevents vasoconstriction in the maternal spiral arteries, ensuring a consistent supply of blood and nutrients to the developing fetus through low-resistance, high-flow circulation.[15]
The clinical signs of pre-eclampsia are linked to widespread endothelial dysfunction, which includes vasoconstriction and end-organ ischemia. This widespread dysfunction may stem from a disparity between angiogenic and anti-angiogenic factors. In women with pre-eclampsia, levels of soluble fms-like tyrosine kinase-1 (sFlt-1) are elevated in both the bloodstream and the placenta compared to those in normal pregnancies. sFlt-1, an anti-angiogenic protein, counteracts the effects of vascular endothelial growth factor (VEGF) and placental growth factor (PIGF), which are both proangiogenic. Similarly, soluble endoglin (sEng) is also found at higher levels in women with pre-eclampsia and shares anti-angiogenic characteristics with sFlt-1.[14]
Both sFlt-1 and sEng are upregulated to some extent in all pregnant women, which supports the notion that hypertensive diseases in pregnancy may be a deviation from normal pregnancy adaptation. Given that natural killer cells play a crucial role in placentation, which requires a degree of maternal immune tolerance for the foreign placenta, it is conceivable that the maternal immune system might react more adversely to certain placentae, such as those that are more invasive than usual. The initial maternal rejection of placental cytotrophoblasts could lead to inadequately remodeled spiral arteries in cases of pre-eclampsia associated with shallow implantation, resulting in downstream hypoxia and the emergence of maternal symptoms due to elevated levels of sFlt-1 and sEng.
Oxidative stress is believed to play a significant role in the development of pre-eclampsia. The primary source of reactive oxygen species (ROS) is the enzyme xanthine oxidase (XO), which is predominantly found in the liver. It is hypothesized that heightened purine catabolism due to placental hypoxia leads to an increase in ROS production within the maternal liver, subsequently releasing into the maternal circulation and causing damage to endothelial cells.
Abnormalities in the maternal immune system and a lack of gestational immune tolerance appear to play significant roles in pre-eclampsia. A notable difference in pre-eclampsia is the shift towards Th1 responses and the production of IFN-γ. The exact source of IFN-γ remains unidentified but may involve uterine natural killer cells, placental dendritic cells influencing T helper cell responses, changes in the synthesis of or response to regulatory molecules, or alterations in the function of regulatory T cells during pregnancy. Aberrant immune responses that promote pre-eclampsia could also stem from altered fetal allorecognition or inflammatory triggers. Increased levels of fetal cells, such as erythroblasts and cell-free fetal DNA, have been observed in the maternal circulation of women who develop pre-eclampsia. This has led to the hypothesis that pre-eclampsia is a disease process initiated by a placental lesion, such as hypoxia, which allows increased fetal material into the maternal circulation, triggering an immune response and endothelial damage, ultimately causing pre-eclampsia and eclampsia.[16]
One theory explaining the susceptibility to pre-eclampsia involves maternal-fetal conflict. This conflict arises when trophoblasts, after the first trimester, invade the mother's spiral arteries to modify them, thus gaining greater access to maternal nutrients. Sometimes, this trophoblast invasion is impaired, leading to insufficient changes in the uterine spiral arteries. It is theorized that the developing embryo emits biochemical signals that cause the mother to develop hypertension and pre-eclampsia, allowing the fetus to benefit from increased maternal blood flow to the compromised placenta. This creates a conflict between the fitness and survival of the mother and fetus, as the fetus prioritizes its own survival and fitness, while the mother has to consider both the current and future pregnancies.
An alternative evolutionary theory suggests that pre-eclampsia may promote pair-bonding and paternal investment in offspring. The condition is hypothesized to be an adaptive mechanism allowing the mother to cease investment in an offspring potentially lacking a supportive father, as indicated by the frequency of the father's semen exposure to the mother. Studies indicate that women exposed more frequently to their partner's semen before conception have a lower risk of pre-eclampsia. Moreover, the risk of pre-eclampsia decreases in subsequent pregnancies with the same father, but increases with a different father.
In pre-eclampsia, the abnormal expression of the chromosome 19 microRNA cluster (C19MC) in placental cell lines diminishes extravillus trophoblast migration. Specific microRNAs within this cluster, such as miR-520h, miR-520b, and miR-520c-3p, may lead to abnormal invasion of the spiral arteries. This results in impaired invasion of extravillus trophoblast cells into the maternal spiral arteries, leading to increased resistance, reduced blood flow, and diminished nutrient supply to the fetus. Tentative evidence suggests that vitamin supplementation may reduce this risk.[17] Additionally, immune factors might contribute to the condition.
Diagnosis[edit | edit source]
Monitoring for pre-eclampsia is advised throughout pregnancy by measuring a woman's blood pressure.
Diagnostic criteria[edit | edit source]
Pre-eclampsia is diagnosed in a pregnant woman when:[18]
- Blood pressure reaches ≥140 mmHg systolic or ≥90 mmHg diastolic on two separate occasions, at least four to six hours apart, after 20 weeks of gestation in someone with previously normal blood pressure.
- For a woman with essential hypertension present before 20 weeks of gestation, the diagnostic criteria include an increase in systolic blood pressure (SBP) of ≥30 mmHg or an increase in diastolic blood pressure (DBP) of ≥15 mmHg.
- Proteinuria is defined as ≥ 0.3 grams (300 mg) or more of protein in a 24-hour urine sample, a SPOT urinary protein to creatinine ratio ≥0.3, or a urine dipstick reading of 1+ or greater (dipstick reading should be used only if other quantitative methods are not available).
Suspicion of pre-eclampsia should be considered in any pregnancy complicated by elevated blood pressure, even without proteinuria. Ten percent of those with other signs and symptoms of pre-eclampsia, and 20% of those diagnosed with eclampsia, may not show proteinuria. In the absence of proteinuria, new-onset hypertension and one or more of the following new-onset conditions may suggest a diagnosis of pre-eclampsia:
- Evidence of kidney dysfunction (oliguria, elevated creatinine levels)
- Impaired liver function (noted by liver function tests)
- Thrombocytopenia (platelet count <100,000/microliter)
- Pulmonary edema
- Ankle edema (pitting type)
- Cerebral or visual disturbances
Pre-eclampsia is a progressive condition, and signs of organ dysfunction suggest severe pre-eclampsia. A systolic blood pressure of 160 mmHg or higher, a diastolic blood pressure of 110 mmHg or higher, and/or proteinuria exceeding 5g in a 24-hour period also indicate severe pre-eclampsia. Clinically, individuals may exhibit symptoms such as epigastric or right upper quadrant abdominal pain, headaches, and vomiting. Severe pre-eclampsia significantly increases the risk of intrauterine fetal death.[19]
It is also important to recognize a rise in baseline blood pressure of 30 mmHg systolic or 15 mmHg diastolic. Although this increase does not meet the absolute diagnostic criteria of 140/90 mmHg, it should not be overlooked.
Differential Diagnosis[edit | edit source]
Pre-eclampsia can be mistaken for various other conditions, including chronic hypertension, renal disease, seizure disorders, gallbladder and pancreatic diseases, immune or thrombotic thrombocytopenic purpura, antiphospholipid syndrome, and hemolytic-uremic syndrome. It should be considered in any pregnant woman past 20 weeks of gestation. Diagnosis is especially challenging when pre-existing conditions like hypertension are present. Women with acute fatty liver of pregnancy may exhibit high blood pressure and proteinuria, but this differs in the degree of liver damage. Other conditions that may cause elevated blood pressure include thyrotoxicosis, pheochromocytoma, and substance misuse.[19]
Complications[edit | edit source]
Complications from pre-eclampsia can impact both the mother and the fetus. In the short term, pre-eclampsia may lead to eclampsia, HELLP syndrome, hemorrhagic or ischemic stroke, liver impairment and dysfunction, acute kidney injury, and acute respiratory distress syndrome (ARDS).[14]
Pre-eclampsia is also linked to a higher occurrence of caesarean sections, preterm births, and placental abruption. Additionally, some individuals may experience a rise in blood pressure during the first postpartum week due to volume expansion and fluid mobilization. For the fetus, there is a risk of growth restriction and the possibility of fetal or perinatal mortality.
In the long term, those who have experienced pre-eclampsia may have an increased risk of its recurrence in future pregnancies.
Eclampsia[edit | edit source]
Eclampsia is the onset of convulsions in a patient with pre-eclampsia that cannot be attributed to other causes. It indicates a severe progression of pre-eclampsia and is linked with high rates of both perinatal and maternal morbidity and mortality. Warning signs of eclampsia in someone with pre-eclampsia may include headaches, visual disturbances, and pain in the right upper quadrant or epigastric area, with headaches being the most common symptom. While brisk or hyperactive reflexes are typical during pregnancy, ankle clonus is a sign of neuromuscular irritability often indicating severe pre-eclampsia, which may also be a precursor to eclampsia.[20] Magnesium sulfate is commonly used to prevent convulsions in severe cases of pre-eclampsia.
HELLP Syndrome[edit | edit source]
HELLP syndrome is characterized by hemolysis (microangiopathic), elevated liver enzymes (indicating liver dysfunction), and low platelet count (thrombocytopenia). It may affect 10–20% of patients with severe pre-eclampsia or eclampsia, leading to higher risks of complications and mortality for both mother and child. Approximately 50% of HELLP syndrome cases occur preterm, 20% in late gestation, and 30% during the postpartum period.[19]
Long term[edit | edit source]
Preeclampsia predisposes individuals to future cardiovascular disease, and a history of preeclampsia or eclampsia can double the risk of cardiovascular mortality later in life. Additional risks include stroke, chronic hypertension, kidney disease, and venous thromboembolism. Preeclampsia and cardiovascular diseases share common risk factors, including age, elevated BMI, family history, and certain chronic conditions. It appears that preeclampsia does not increase the risk of cancer.[21]
Reduced blood flow to the fetus in cases of pre-eclampsia can lead to a decreased supply of nutrients, potentially resulting in intrauterine growth restriction (IUGR) and low birth weight. The fetal origins hypothesis suggests that fetal undernutrition is associated with an increased risk of coronary heart disease in later adult life, due to imbalanced growth.[22]
Pre-eclampsia can cause a disparity between the energy provided by the mother and the energy required by the fetus, leading to Intrauterine Growth Restriction (IUGR) in the developing fetus. Infants with IUGR may experience suboptimal neuronal development and are at an increased risk for adult diseases, as suggested by the Barker hypothesis. The range of adult diseases associated with IUGR in the fetus includes coronary artery disease (CAD), type 2 diabetes mellitus (T2DM), cancer, osteoporosis, and various psychiatric disorders.[23]
The likelihood of pre-eclampsia and the emergence of placental dysfunction have been demonstrated to recur across generations, particularly on the maternal side and likely on the paternal side as well. Offspring born to mothers who were small for gestational age (SGA) have a 50% increased risk of developing pre-eclampsia, while those born to both SGA parents have a threefold higher risk of experiencing pre-eclampsia in subsequent pregnancies.[24]
Postpartum preeclampsia[edit | edit source]
Preeclampsia may also manifest in the postpartum period, following delivery. While definitive guidelines for postpartum preeclampsia are not established, it is suggested that new-onset preeclampsia can develop from 48 hours to up to 6 weeks post-delivery.[25]
The diagnostic criteria for postpartum preeclampsia are essentially the same as those for preeclampsia diagnosed during pregnancy. Likewise, many risk factors are similar; however, a lack of prior pregnancy does not appear to be a risk factor for postpartum preeclampsia.[26] Other risk factors are associated with labor and/or delivery, such as cesarean delivery and higher rates of intravenous fluids.
The American College of Obstetricians and Gynecologists advises evaluating blood pressure in patients with any hypertensive disorders of pregnancy within 7-10 days post-delivery. Home monitoring of blood pressure may enhance the chances of obtaining measurements within these suggested intervals.[27]
Generally, the treatment for postpartum preeclampsia mirrors that during pregnancy, which includes the administration of anti-hypertensive drugs to reduce blood pressure and magnesium sulfate to avert eclampsia. The blood pressure medications prescribed during pregnancy are also applicable postpartum. Once the concern for the fetus is no longer present, other medications may be considered. Typically, ACE inhibitors, beta-blockers, and calcium channel blockers are deemed safe for breastfeeding patients. Currently, there is no conclusive data favoring any particular medication for managing postpartum blood pressure. Moreover, evidence suggests that the diuretic furosemide could potentially reduce the duration of hypertension in those with postpartum preeclampsia.[27]
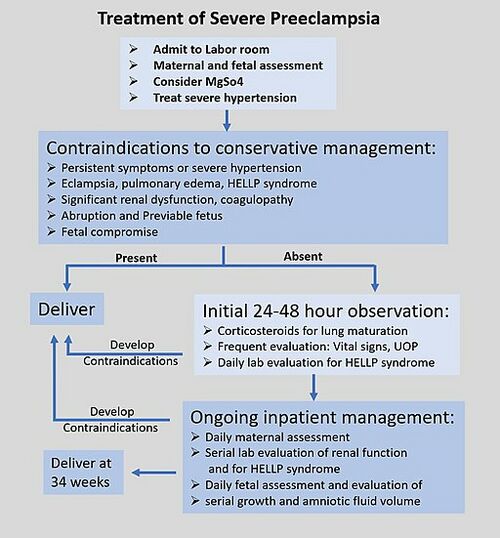
Treatment[edit | edit source]
Preeclampsia may happen at any time during the second half of pregnancy or the first few days after the birth. The signs of preeclampsia are high blood pressure, protein in urine and sudden excessive swelling of the face, hands and feet. Sudden blurred vision is also a symptom. It is also possible to have preeclampsia without having any symptoms at all.
Women seen as high-risk individuals for developing preeclampsia should have administration of low-dose aspirin on a daily basis from early pregnancy as this is currently the most effective way to prevent preeclampsia. For preeclamptic females, specific information and counseling should be provided and appointments with a specialist made. In pregnancies complicated with preeclampsia, closer monitoring and antepartum surveillance ( eg.Doppler ultrasound blood flow study, biophysical profile, non-stress test, and oxytocin challenge test). Early intervention and aggressive therapy should be considered if tests indicate this action. [28]
Maternal Consquences[edit | edit source]
Preeclampsia is associated with:
- An increased relative risk for the development of end-stage kidney disease (ESKD) in the mother.
- Research shows that women with a history of preeclampsia are 60% more likely to experience ischemic stroke and also have an increased risk of hemorrhagic stroke and venous thromboembolism[29].
- Women with a history of preeclampsia have increased an increased risk of cerebral small vessel disease that is highly associated with stroke and dementia. [30]
Neonatal Consequences[edit | edit source]
The neonatal outcomes of preeclampsia identified are:
- Preterm birth, stillbirth, low birth weight (LBW), low Apgar score, intrauterine growth reduction (IUGR), neonatal intensive care unit (NICU) admission.[31]
- Other differences include delayed child physical development and sensorimotor reflex maturation( see Stages of Cognitive Development) , increased body mass index, changes in neuroanatomy and reductions in cognitive function, and hormonal changes.[29]
Physiotherapy[edit | edit source]
Women with higher levels of physical activity(PA) during pregnancy seem to have a lower risk of preeclampsia, conversely women with increased levels of sedentary activity were at increased risk. Promotion of PA during pregnancy may be a promising approach for reducing the risk of the disease. However, additional research is needed to confirm the protective effect of PA on preeclampsia risk.[5]
In 2012 the American College of Obstetricians and Gynecologists does not recommend exercise therapy during pregnancy for women at risk of certain gestational complications, such as gestational hypertension and preeclampsia. Research suggests that exercise therapy has deleterious effects on uteroplacental perfusion in at-risk pregnancies. Although several studies observed a beneficial effect of exercise therapy on preeclampsia risk, they were not randomized studies and the mechanisms involved in these effects are unknown.[32]
References[edit | edit source]
- ↑ Eiland E, Nzerue C, Faulkner M. Preeclampsia 2012. Journal of pregnancy. 2012;2012.
- ↑ Laule CF, Odean EJ, Wing CR, Root KM, Towner KJ, Hamm CM, Gilbert JS, Fleming SD, Regal JF. Role of B1 and B2 lymphocytes in placental ischemia-induced hypertension. American Journal of Physiology-Heart and Circulatory Physiology. 2019 Oct 1;317(4):H732-42.
- ↑ Al-Jameil N, Khan FA, Khan MF, Tabassum H. A brief overview of preeclampsia. Journal of clinical medicine research. 2014 Feb;6(1):1.
- ↑ Martin N. Trusted Health Sites Spread Myths About a Deadly Pregnancy Complication.
- ↑ 5.0 5.1 Spracklen CN, Ryckman KK, Triche EW, Saftlas AF. Physical activity during pregnancy and subsequent risk of preeclampsia and gestational hypertension: a case control study. Maternal and child health journal. 2016 Jun;20:1193-202.
- ↑ Parker SE, Werler MM, Gissler M, Tikkanen M, Ananth CV. Placental Abruption and Subsequent Risk of Pre‐eclampsia: A Population‐Based Case–Control Study. Paediatric and perinatal epidemiology. 2015 May;29(3):211-9.
- ↑ Opichka MA, Rappelt MW, Gutterman DD, Grobe JL, McIntosh JJ. Vascular dysfunction in preeclampsia. Cells. 2021 Nov 6;10(11):3055.
- ↑ Karrar SA, Hong PL. Preeclampsia. InStatPearls [Internet] 2023 Feb 13. StatPearls Publishing.
- ↑ Phipps EA, Thadhani R, Benzing T, Karumanchi SA. Pre-eclampsia: pathogenesis, novel diagnostics and therapies. Nature Reviews Nephrology. 2019 May;15(5):275-89.
- ↑ Bartsch E, Medcalf KE, Park AL, Ray JG. Clinical risk factors for pre-eclampsia determined in early pregnancy: systematic review and meta-analysis of large cohort studies. Bmj. 2016 Apr 19;353.
- ↑ Garg AX, Nevis IF, McArthur E, Sontrop JM, Koval JJ, Lam NN, Hildebrand AM, Reese PP, Storsley L, Gill JS, Segev DL. Gestational hypertension and preeclampsia in living kidney donors. New England Journal of Medicine. 2015 Jan 8;372(2):124-33.
- ↑ van den Boogaard E, Vissenberg R, Land JA, van Wely M, van der Post JA, Goddijn M, Bisschop PH. Significance of (sub) clinical thyroid dysfunction and thyroid autoimmunity before conception and in early pregnancy: a systematic review. Human reproduction update. 2011 Sep 1;17(5):605-19.
- ↑ Vissenberg R, Van den Boogaard E, Van Wely M, Van der Post JA, Fliers E, Bisschop PH, Goddijn M. Treatment of thyroid disorders before conception and in early pregnancy: a systematic review. Human reproduction update. 2012 Jul 1;18(4):360-73.
- ↑ 14.0 14.1 14.2 Mustafa R, Ahmed S, Gupta A, Venuto RC. A comprehensive review of hypertension in pregnancy. Journal of pregnancy. 2012;2012.
- ↑ Chen DB, Wang W. Human placental microRNAs and preeclampsia. Biology of reproduction. 2013 May 1;88(5):130-.
- ↑ Laresgoiti-Servitje E, Gomez-Lopez N, Olson DM. An immunological insight into the origins of pre-eclampsia. Human reproduction update. 2010 Sep 1;16(5):510-24.
- ↑ Fu ZM, Ma ZZ, Liu GJ, Wang LL, Guo Y. Vitamins supplementation affects the onset of preeclampsia. Journal of the Formosan Medical Association. 2018 Jan 1;117(1):6-13.
- ↑ Hauser S, Longo DL, Jameson JL, Kasper DL, Loscalzo J, editors. Harrison's Principles of Internal Medicine: V. 1. McGraw-Hill Companies, Incorporated; 2012.
- ↑ 19.0 19.1 19.2 Arulkumaran N, Lightstone L. Severe pre-eclampsia and hypertensive crises. Best Practice & Research Clinical Obstetrics & Gynaecology. 2013 Dec 1;27(6):877-84.
- ↑ Anthony J, Damasceno A, Ojjii D. Hypertensive disorders of pregnancy: what the physician needs to know: review articles. Cardiovascular journal of Africa. 2016 Mar 1;27(2):104-10.
- ↑ Bellamy L, Casas JP, Hingorani AD, Williams DJ. Pre-eclampsia and risk of cardiovascular disease and cancer in later life: systematic review and meta-analysis. Bmj. 2007 Nov 8;335(7627):974.
- ↑ Barker DJ. Fetal origins of coronary heart disease. Bmj. 1995 Jul 15;311(6998):171-4.
- ↑ Calkins K, Devaskar SU. Fetal origins of adult disease. Current problems in pediatric and adolescent health care. 2011 Jul 1;41(6):158-76.
- ↑ Wikström AK, Svensson T, Kieler H, Cnattingius S. Recurrence of placental dysfunction disorders across generations. American journal of obstetrics and gynecology. 2011 Nov 1;205(5):454-e1.
- ↑ Hauspurg A, Jeyabalan A. Postpartum preeclampsia or eclampsia: defining its place and management among the hypertensive disorders of pregnancy. American journal of obstetrics and gynecology. 2022 Feb 1;226(2):S1211-21.
- ↑ Redman EK, Hauspurg A, Hubel CA, Roberts JM, Jeyabalan A. Clinical course, associated factors, and blood pressure profile of delayed-onset postpartum preeclampsia. Obstetrics & Gynecology. 2019 Nov 1;134(5):995-1001.
- ↑ 27.0 27.1 Steele DW, Adam GP, Saldanha IJ, Kanaan G, Zahradnik ML, Danilack VA, Stuebe AM, Peahl AF, Chen KK, Balk EM (2023-05-31). Management of Postpartum Hypertensive Disorders of Pregnancy
- ↑ Chang KJ, Seow KM, Chen KH. Preeclampsia: recent advances in predicting, preventing, and managing the maternal and fetal life-threatening condition. International journal of environmental research and public health. 2023 Feb 8;20(4):2994.
- ↑ 29.0 29.1 Turbeville HR, Sasser JM. Preeclampsia beyond pregnancy: long-term consequences for mother and child. American Journal of Physiology-Renal Physiology. 2020 Jun 1;318(6):F1315-26.
- ↑ Miller EC. Preeclampsia and cerebrovascular disease: the maternal brain at risk. Hypertension. 2019 Jul;74(1):5-13.Available:https://www.ahajournals.org/doi/10.1161/HYPERTENSIONAHA.118.11513 (accessed 5.3.2024)
- ↑ Atamamen TF, Naing NN, Oyetunji JA, Wan-Arfah N. Systematic literature review on the neonatal outcome of preeclampsia. Pan African Medical Journal. 2022 Jan 31;41(1).
- ↑ Genest DS, Falcao S, Gutkowska J, Lavoie JL. Impact of exercise training on preeclampsia: potential preventive mechanisms. Hypertension. 2012 Nov;60(5):1104-9.Available:https://www.ahajournals.org/doi/10.1161/HYPERTENSIONAHA.112.194050 (accessed 6.3.2024)