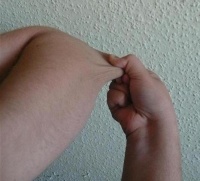
Ehlers-Danlos Syndrome
Original Editors - Corey Vogt from Bellarmine University's Pathophysiology of Complex Patient Problems project.
Top Contributors - Corey Vogt, Eliza Clark, Megan Kanter, Admin, Lucinda hampton, Elaine Lonnemann, Kim Jackson, Laura Ritchie, Abbey Wright, 127.0.0.1, Rosie Swift, Wendy Walker and Blessed Denzel Vhudzijena - Your name will be added here if you are a lead editor on this page.
Definition/Description [edit | edit source]
Ehlers-Danlos syndrome is a hereditary collagen disorder characterized by articular hypermobility, dermal hyperelasticity, and widespread tissue fragility.[1] Individuals with EDS demonstrate defects in the body's connective tissues, manifesting as altered strength, elasticity, integrity, and healing properties of the tissues. The severity of the syndrome varies greatly depending upon the specific mutation. However each type demonstrates some degree of integumentary involvement and/or joint hypermobility/laxity.[2]
Ehlers Danlos Syndrome traces its initial discover date and description to the fourth century BC. The first clinical description of EDS is credited to Tschernogobow in 1892. However, the name and recognition of the syndrome is credited to Edward Ehlers, a Danish dermatologist, and Henri-Alexandre Danlos, a French physician, both of who wrote separate reports in 1901 and 1908 respectively. The two physicians were able to collaborate in providing a description of the pertinent features of the condition as well as accurately identify the associated phenotypes.[2]
In 1997-1998, six discernable phenotypes for EDS were identified (classic, hypermobility, vascular, kyphoscoliosis, arthrochalasia, and dermatosparaxis) by Beighton et. al. These identifiable forms of EDS are currently recognized by the medical advisory board of the Ehlers-Danlos National Foundation and used in the clinical setting for proper diagnosis.[2] All of these phenotypes of EDS can lead to significant disability and decreased quality of life for the individual. The disability is multidimensional including physical impairments, chronic pain, fatigue, maladaptive cognition, and psychological stress.[3]
Prevalence[edit | edit source]
Combined prevalence of all subtypes of EDS is about 1 per 5,000. Hypermobility and classic subtypes are the most common with a prevalence of 1 per 10,000-15,000 and 1 per 20,000-40,000 respectively. EDS demonstrates equal prevalence amongst males and females of all racial and ethnic backgrounds. [2][4][5][6][7]
There are other forms of EDS that are very rare. These include the arthrochalasia, kyphoscoliosis, dermatosparaxis, and vascular types. Currently, only 30 cases of the arthrochalasia and 60 cases of kyphoscoliosis have been reported worldwide. The only cases of dermatosparaxis type have been seen in infants and children, and has only been found in a dozen individuals. The vascular type is the rarest form, affecting about 1 in 250,000 people worldwide. [8]
Characteristics/Clinical Presentation[edit | edit source]
Ehlers-Danlos Syndrome contains at least six discernible phenotypes that are individually recognized. However, each type contains characteristics similar to the others, often proving problematic in accurate diagnosis. Despite the frequent overlap of associated signs and symptoms of the various subtypes of EDS, each specific type presents with the same general clinical characteristics that are a result of faulty or reduced amounts of Type III collagen in the body:[2][5][4][6][7][9]
- Hyperextensible (stretchy) skin
- Tissue fragility
- Poor wound healing resulting in elongated scarring (cigarette paper scars)
- Joint hypermobility
- Increased propensity for joint subluxations/disclocations
- Muscle weakness
- Delayed motor development
- Easy bruising
- Fatigue
- Frequent clumsiness
- Gait defects
- Frequent falls
- Poor coordination[10]
- Possible developmental delay
Chronic Pain may be present in patients with the Hypermobility Type of Ehlers-Danlos.[11]
In 1997-1998, Beighton et al. in collaboration with the Ehlers-Danlos National Foundation proposed a system to distinguish the clinical manifestations of EDS into six distinct subtypes. This nosology is still being used in the clinical setting to achieve accurate diagnosis of EDS.[2][4][5][6][7][12]
| Type | Inheritance | Previous Nomenclature | Major Features | Minor Features | Laboratory |
| Classic[5][12] | AD | Type I/II |
Skin hyperextensibility (atrophic scarring) Joint hypermobility |
Smooth velvety skin Easy bruising Molluscoid pseudotumors Subcutaneous spheroids Joint hypermobility complications (sprains, subluxations, dislocations) Muscle hypotonia Delayed gross motor development Tissue extensibility and fragility complications (hiatal hernia, anal prolapse, cervical insufficiency) Postoperative hernia Positive family history |
Abnormalities in skin collagen assessed under electron microscopy Abnormal type V collagen - 30% due to mutation in tenascin |
| Hypermobility[4][12] | AD | Type III |
Skin involvement (hyperextensibility and/or smooth, velvety skin) Generalized joint hypermobility Chronic Pain[11] |
Recurring joint dislocations Chronic joint/limb pain Positive family history |
|
| Vascular[7][12] | AD | Type IV |
Thin, translucent skin Arterial/intestinal/uterine fragility or rupture Extensive bruising Characteristic facial appearance |
Acrogeria Hypermobility of small joints Tendon/muscle rupture Talipes equinovarus Early onset varicose veins Arteriovenous, carotid-cavernous sinus fistula Pneumothorax/pneumohemothorax Gingival recession Positive family history Sudden death in close relatives |
Abnormal type III collagen COL3AI mutation |
| Kyphoscoliosis[6][12] | AR | Type VI |
General joint hypermobility Severe muscle hypotonia at birth Progressive scoliosis present from birth Scleral fragility or rupture of ocular globe |
Tissue fragility (atrophic scarring); eary bruising Arterial rupture Marfan-like habitus Microcornea Osteopenia as defined radiologically Positive family history |
Urinalysis for lysylpyridinoline and hydroxylysylpyridinoline |
| Arthrochalasia[12] | AD | Type VII A/B |
Severe generalized joint hypermobility Recurrent joint subluxations Congenital bilateral dislocated hips |
Skin hyperextensibility Tissue fragility (atrophic scarring) Easy bruising Muscle hypotonia Kyphoscoliosis Mild osteopenia as defined radiologically |
Skin biopsy and demonstration of abnormal type I collagen |
| Dermatosparaxis[12] | AR | Type VII C |
Severe skin fragility Sagging, redundant skin |
Soft, doughy skin texture Easy bruising Premature rupture of fetal membranes Large hernias (inguinal and umbilical) |
Demonstration of abnormal type I collagen chains in skin |
AD = Autosomal Dominant
AR = Autosomal Recessive
Associated Co-morbidities[edit | edit source]
Many different medical conditions/disease states occur in individuals with EDS. Examples of co-morbidities include:[4][2][10]
- Gastroesophageal reflux
- Gastritis
- Irritable Bowel Sydrome
- Autonomic Dysfunction (neurally mediated hypotension, postural orthostatic tachycardia syndrome, paroxysmal supraventricular tachycardia)
- Aortic root dilatation
- Mitral valve prolapse
- Periodontal disease (friability, gum disease, gum recession)
- Temporomandibular Joint dysfunction
- Depression
- Developmental coordination disorder (in children)
Medications[2][4][edit | edit source]
Analgesics - pain relief
- Acetaminophen
- Tramadol
- Lidocaine
- Tricyclic antidepressants
- Serotonin/norepinephrine receptor inhibitors
- Opioids
NSAIDS - anti-inflammatory effect
- Ibuprofen, naproxen
- Cox-2 Inhibitors
- Corticosteriod injections (pain and inflammation)
Muscle relaxants - treatment of myofascial spasms
Glucosamine and Chondroitin - treatment of osteoarthritis
Supplemental magnesium/potassium - muscle relaxation and pain relief
Vitamin C - enhancement of wound healing and proliferation of collagen synthesis
Diagnostic Criteria[edit | edit source]
Clinical Examination and a detailed family history have proven to be the most effective means of accurately diagnosing EDS.
Major diagnostic criteria typically includes:[2][4][10]
- Joint hypermobility as indicated by a score of greater than or equal to 6/9 on the Beighton scale (Gold standard)
- Soft skin or skin hyperextensibility as defined by >1.5 cm on volar surface of forearm
- Fragile skin or significant skin/soft tissue abnormalities (easy bruising, delayed wound healing, atrophic scarring, easy tendon, ligament, vessel rupture)
Minor diagnostic criteria typically includes:[2][4]
- Positive family history
- Recurring joint subluxations/dislocations
- Chronic joint, limb, or back pain
- Altered blood pressure responses (neurally mediated hypotension or postural orthostatic tachycardia)
- Functional bowel disorders
- High, narrow palate
- Dental crowding
Laboratory Tests/Laboratory Values[edit | edit source]
Laboratory studies can be utilized as supporting evidence to confirm the diagnosis of a specific subtype of EDS.
Biochemical Studies can be used to analyze the make-up of collagen molecules in cultured skin fibroblasts and detect alterations to support a diagnosis of EDS:[2][4]
- Type IV EDS - Vascular
- Type VIIA and VIIB EDS- Arthrochalasia
- Type VIIC - Dermatosparaxis
Molecular testing utilizing DNA analysis can be used in diagnosis of EDS:[2][4]
- Type IV - Vascular
- Type VII - Arthrochalasia/Dermatosparaxis
Urinary Analyte Assay can be used in diagnosis of EDS:[2][4]
- Type VI - Kyphoscoliotic
Diagnostic Tests[edit | edit source]
CT scanning, MRI scanning, ultrasonography, electrocardiograms, and angiography are useful in diagnosing Type IV (Vascular) EDS with reports suggesting the presence of arterial aneurysms, arterial dissections, arterial ectasias, and arterial occlusions.[2][4]
Ultrastructural examination of collagen fibrils has been utilized as an assistive tool for diagnosis of Type I/II (Classical) EDS and Type VIIA/Type VIIB ( Arthrochalasia) EDS.[2][4]
Skin Biopsy using histopathologic analysis has yet to be proven as beneficial in the diagnostic process of EDS.[2][4]
Causes[edit | edit source]
EDS is classified as an inherited connective tissue disease. These patients’ tissues have greater amount of procollagen than normal and have a defect in the conversion of procollagen to collagen.[13] Three patterns of inheritance have been linked with the various subtypes of EDS: autosomal dominant, autosomal recessive, and X-linked (rarest form). The exact source of genetic mutation responsible for the condition is unknown. However, mutations in ADAMTS2, COL1A1, COL1A2, COL3A1, COL5A1, COL5A2, PLOD1, and TNXB genes have been linked to causation of EDS.[2][4]
- COL1A1, COL1A2, COL3A1, COL5A1, COL5A2 encode the manufacture of proteins that are responsible for multiple types of collagen
- ADAMTS2, PLOD1, and TNXB encode the manufacture of proteins that interact with or process collagen
Patients who have the vascular type EDS also lack type III collagen. Type III collagen is fibrillation forming collagen of 3 alpha-1 chains and is the most abundant type of collagen in the human body. In adults, it makes up the majority of the extracellular matrix in internal organs, especially the cardiovascular system and skin, resulting in arterial and vein complications. Sudden death has been reported in some cases.[14]
Systemic Involvement[2][4][3][10][edit | edit source]
Musculoskeletal
- Joint laxity manifesting as recurrent joint subluxations/dislocations due to minimal trauma and/or spontaneous onset. Joints involved typically include the vertrebral column, costo-vertebral, costo-sternal articulations, temporomandibular and joints of the extremities.
- Augmented biomechanics which results in less effective muscle contraction.
- Osteoarthritis resulting in early onset of degenerative joint disease. Early onset OA associated with increased mechanical stress placed on joints resulting from extreme ligamentous and articular laxity.
- Osteopenia due to reduction in general bone density. Precursor and predisposition to early onset of osteoporosis due to abnormally low bone density.
- Osteoporosis due to reduction in general bone bone density up to 0.9 standard deviations lowering than the average, healthy adult
- Scoliosis
- Kyphosis
- Chronic joint, ligament, tendon, or muscle pain due to myofascial and/or neuropathic source
- Headaches related to muscle tension in cervical spine and TMJ dysfunction
Neuromuscular
- Low muscle tone (hypotonia)
- Generalized muscle weakness-- more likely due to a muscle dysfunction than atrophy and muscle loss
- Decreased reflexes in the knee extensors and flexors seen in adolescents
Neurological
- Fatigue, pain and anxiety are often due to exhaustion of the CNS's reserves
- Migraines often disabling
- Chronic pain results from the tight link of peripheral biomechanic dysfunctions and CNS fatigue. This often leads to fear of movement that is aggravated with activity, ultimately leading to muscular deconditioning.
- Hyperalgesia is commonly seen in children and adults with hypermobility EDS due their central nervous system being highly sensitized
Cardiopulmonary
- Dysautonomia or Autonomic Dysfunction resulting in abnormal chest pain, palpitations at rest or with exertion, or abnormal blood pressure responses. Condition can be manifested as premature atrial complexes, paroxysmal supraventricular tachycardia, neurally mediated hypotension (NMH), or postural orthostatic tachycardia syndrome (POTS). Occurs in 33-50% of individuals with EDS, especially hypermobility and classic subtypes
- Aortic Root Dilation resulting in predisposition to arterial fragility or rupture. Typically occurs in a mild form in 25-33% of individuals with hypermobility and classic subtypes of EDS. Appears to be less severe than found in Marfan's Syndrome displaying no increased risk of dissection unless a prominent dilatation is present. Places individual at an increased risk for development of an abdominal aortic aneurysm (AAA).
- Mitral Valve Prolapse with increased risk of developing infective endocarditis
Gastrointestinal
- Functional Bowel Disorders (gastritis, irritable bowel syndrome, gastroesophageal reflux) occur in up to 50% of individuals with EDS
- High prevalence of GI reflux abdominal pain, constipation and diarrhea in adolescents with hypermobility EDS
Integumentary
- Hyperextensibility of skin
- Fragility of soft tissue resulting in increased likelihood of rupture or tearing of internal organs
Genitourinary
- Uterine Fragility
- Premature rupture of fetal membranes during pregnancy
- Pelvic prolapse
- Dyspareunia
- Urinary incontinence often seen in adolescents with Type III
Oral/Dental
- Periodontal disease resulting in friability, gingivitis, and gum recession
- Presence of a high, narrow palate combined with dental crowding
Hematologic
- Easy bruising
- Prolonged bleeding times, epistaxis, and menometrorrhagia
Psychiatric
- Depression
Medical Management (current best evidence)[2][4][15][edit | edit source]
Currenly Ehlers-Danlos Syndrome has no cure. Treatment and management of the condition includes a combination of prevention, management, and education about the specific characteristics of the syndrome as well as how to avoid primary and secondary manifestations of the condition. Presently a specific treatment protocol does not exist due to the large variability of signs and symptoms present in affected individuals and amongst the various subtypes of EDS. Each specific treatment protocol is individually designed and specialized for the affected individual in order to meet the needs of that specific patient.
Treatment of EDS typically consists of management of specific signs and symptoms of the condition as well as lifestyle adjustments to prevent injury/complications. Medical management is usually overseen by a physician specializing in physiatry/physical medicine and rehabilitation (PM&R). Referral sources include a physical therapist, occupational therapist, dentist, ophthalmologist, and genetic counselor to provide the patient with a comprehensive and holistic treatment approach.
A recent study on women with hypermobility type EDS showed significant improvement in performance, performance satisfaction, and decreased kinesiophobia when provided with an intensive multidisciplinary rehabilitation program. This included a cognitive-behavioral approach with strength and endurance training and pain coping.
Education
- Avoidance of high impact activities that place increased stress on pre-morbid lax joints, such as heavy lifting or resistance training
- Avoidance of activities that require joint hyperextension, such as excessive stretching or repetitive activities
- Meticulous skin care
- Meticulous dental care
- Frequent medical check-ups for vascular dysfunction associated with Vascular EDS, bone density (DEXA scans), or orthopaedic dysfunctions associated with increased joint laxity and low muscle tone
Physical Therapy
- Exercise program consisting of aerobic conditioning combined with a low resistance, high repetition resistive training program to promote increased joint stability by increasing general resting muscle tone
- Assistive devices to provide loading relief to lower extremity joints during ambulation and weight bearing activities
- Bracing to promote increased joint stability and decrease likelihood of joint subluxation/dislocation
- Pain management techniques to address soft tissue, myofascial, and chronic joint pain associated with EDS
- Safe, effective, efficient transfers to avoid excessive weight bearing or loading of lower extremity joints
Occupational Therapy
| [16] |
- Bracing/splinting in combination with orthopaedists, rheumatologists, and physical therapists to promote increased joint stability and decrease likelihood of joint subluxation/dislocation, especially in upper extremity joints and vertebral joints
Ophthalmologist
- Consultation to screen for myopia, retinal tears, and keratoconus common in individuals with EDS
Dentist
- Consultation to screen for periodontitis and to emphasize importance of meticulous dental care in individuals with EDS
Surgical/Invasive Procedures
Surgical and/or other invasive procedures are not necessarily recommended in patients with EDS as a means of primary treatment due to the impaired wound healing, increased likelihood of scarring, and increased likelihood of blood vessel rupture associated with EDS. However, certain subtypes of EDS, most notably the classic and vascular subtypes of EDS possess an increased predisposition to surgical complications compared to the others.
| [17] |
- Arthroscopic debridement, tendon relocations, capsulorraphy, and arthroplasty have been performed on individuals with EDS with degree of stabilization, pain relief, patient satisfaction, and overall improvements being variable and less than individuals without EDS
- Prolotherapy - A procedure, in which saline/other irritants are injected into tendons or surrounding joints to produce inflammation and subsequent scar formation in hopes of creating increased soft tissue stability
- Anesthetic/corticosteroid injections - A procedure designed to address acute, localized areas of pain and inflammation by injecting anti-inflammatory solutions/medications
- Anesthetic nerve blocks - A procedure, in which an injection occurs to specific nerve using anesthetic medication to provide temporary pain relief resulting from a neuropathic origin
- Intrathecal anesthesia/opioid medication - A constant delivery of numbing/pain medication to address the presence of chronic pain and to reduce the need for oral/systemic medications
Physical Therapy Management (current best evidence)[2][4][3][18][10][19][edit | edit source]
Unfortunately, no set protocol of physical therapy interventions exists to address the impairments and functional limitations associated with EDS, due largely in part to the varied presentation of the condition for each affected individual. Therefore, each physical therapy plan of care must be specially created for the patient depending upon the subtype of EDS and the signs and symptoms of that patient.
In general, physical therapy intervention focuses on decreasing the patient’s disability from a multidirectional approach. This includes ADLs, ambulation, sports activities, and quality of life. Functional activities and therapeutic exercises will be aimed at increasing joint stability through proprioceptive training, gentle stretching, resistance training and use of adaptive equipment to accomplish ADLs. It will be crucial to monitor vitals throughout treatment, especially blood pressure, in this population. This is particularly important in the subtypes that involve the cardiovascular system.
A recent systematic review presented the evidence-based rationale for physical therapy treatment of children, adolescents, and adults diagnosed with joint hypermobility syndrome/hypermobile Ehlers Danlos syndrome. Simmonds et al. conclude that PT's "play an important role in management through exercise prescription and patient education for many of these conditions". However, more robust high quality research needs to be performed in this patient population for assessment of conservative and surgical management[20].
Outcome Measures
There is currently not adequate research for specific EDS outcomes. However, these are some of the suggested assessment tools used to measure progress of impairments in this population. It is possible many different measurements could be used that focus on balance, gait speed, cadence, dual-task activities, quality of life, and fear of falling.
- Activities-specific Behavior Confidence
- Falls Efficacy Scale
- mCTSIB
- DGI/FGA
- Multidimensional Fatigue Symptom Inventory
Resistance training: to avoid recurrent joint subluxations/dislocations due to increased muscle tone and to counteract presence of excessive joint, ligament, tendon, and muscle laxity
- Low resistance, high repetition activities
- Goal is to improve static and dynamic muscle tone to promote increased joint stability during weightbearing and functional activities
- Incorporate core stabilization exercises early rehab and progress throughout
- Prevent excessive loading through weight bearing joints
- Avoid excessive use of involved joints for heavy lifting
- Strengthening the shoulder girdle is important in patients with kyphoscoliosis.
- Past studies have shown that strengthening the knee in the full hypermobile range rather than just the neutral range is more beneficial to improve strength, pain, and impaired function.
Proprioceptive Exercises
- Closed kinetic chain exercises
- Balance
- Plyometric training
Aerobic training
- Walking
- Bicycling
- Low-impact aerobics and/or water aerobics
- Swimming, particularly type VI
- Goal is to promote increased static and dynamic muscle tone to prevent acute joint subluxations/dislocations by minor trauma or stimulus
- May function as pain relief mechanism for individuals experiencing chronic joint and muscle pain associated with EDS
Myofascial release techniques
- Pain relief (immediate - several hours)
- Allows pain free participation in resistance training or daily activities
- Goal is to reduce the presence of muscle spasms that result in intense pain in muscles and surrounding ligaments, tendon, and joints
Modalities
- Hot/cold pack
- Massage
- Ultrasound
- Electrical stimulation
- Acupuncture
- Acupressure
- Goal is to provide pain relief to the patient, who may/may not be experiencing chronic muscle and joint pain from frequent joint subluxations/dislocations, myofascial spasms, and trigger points associated with EDS
- Selection of proper modality is dependent upon patient preference
Adaptive Equipment
- Wheelchair/scooter
- Walker/Crutches/Cane (should be used with caution and discretion due to increased weight bearing through upper extremities with use)
- Modified eating utensils (prevents excessive strain placed on small joints of hands and fingers)
- Modified writing utensils (prevents excessive strain placed on small joints of hands and fingers)
- Modified sleeping surface (air mattress, viscoelastic foam mattress, pillow mattress)
- Goal is to allow daily functioning and promote increased quality of life by decreasing pain or chance of joint subluxation/dislocation
According to Woinarosky “the reported prevalence of JHS/hEDS in adult physical therapy outpatient musculoskeletal settings has been reported to be between 30% and 55%."[10] Despite diagnostic differences between Hypermobility Syndrome and genetic disorders (characterized by generalized joint hypermobility), such as Ehlers-Danlos Syndrome, similar treatment approaches and interventions remain relevant and appropriate between the two diagnostic categories. Russek advocates the use of education and exercise as potential interventions for Hypermobility Syndrome. Education on ergonomics and body mechanics may result in decreases in musculoskeletal pain as well as assist in joint protection strategies. Splints, braces, and taping may also function as viable options to protect vulnerable joints. Russek suggests that therapeutic exercises, such as strengthening, proprioceptive activities, balance, and coordination to affected and surrounding joints as a means for treatment of Hypermobility Syndrome.[21][22]
Differential Diagnosis[4] [edit | edit source]
Marfans Syndrome
- Characterized by additional skeletal, ocular, cardiovascular, pulmonary, and integumentary signs and symptoms beyond those characteristic of EDS. Mimics hypermobility subtype of EDS, but clinical diagnosis is confirmed by the presence of a mutation in the FBN1 gene.
Loeys-Dietz Syndrome
- Characterized by multiple arterial aneurysms and tortuosity. Other clinical signs and symptoms include ocular hypertelorisma and a bifid uvula. Mimics vascular subtype of EDS, but clinical diagnosis is confirmed by detection of a mutation in the TGFBR1 or TGFBR2 gene.
Stickler Syndrome
- Characterized by sensorineural hearing loss, vitreoretinal abnormalities, and cleft palate. Clinical diagnosis is often based on the presence of clinical features, but the syndrome has been associated with mutations in one of three genes (COL2A1, COL11A1, or COL11A2)
Williams Syndrome
- Characterized by a gene deletion resulting in cardiovascular disease (elastin arteriopathy, peripheral pulmonary stenosis, supravalvular aortic stenosis, and hypertension), distinctive facies, connective tissue abnormalities, mental retardation, a specific cognitive profile including personality, growth abnormalities, and endocrine abnormalities (hypercalcemia, hypercalciuria, hypothyroidism, and early puberty). Clinical diagnosis consists of the presence of a contiguous gene deletion of the Williams-Beuren syndrome critical region (WBSCR) that controls the elastin gene.
Aarskog-Scott Syndrome
- Characterized by a shawl scrotum, widow's peak, short upturned nose, other dysmorphic features, and occasionally mental retardation. Clinical diagnosis consists of presence of a mutation of the FGD1 gene.
Fragile X Syndrome
- Not commonly confused with EDS, but does share some characteristics similar to EDS such as joint laxity and EDS-like skin abnormalities. In affected males, characterized by large head, long face, prominent forehead and chin, protruding ears, joint laxity, large testes, and moderate retardation. In affected females, characterized by mild retardation. Clinical diagnosis consists of the presence of a mutation of the FMR1 gene.
Achondroplasia/hypochondroplasia
- Characterized by short stature with distinguished skeletal features. Clinical diagnosis consists of characteristic clinical and radiographic findings in 70-99% of affected individuals as well as the presence of a mutation in the FGFR3 gene.
- Characterized by the presence of multiple fractures and in some cases, dentinogenesis imperfecta (grey or brown teeth). Biochemical testing reveals the presence of abnormalities in structure and quantity of type I collagen (98% of type II OI, 90% of type I OI, 84% of type IV OI, 84% of type III OI). About 90% of individuals with Type I-IV OI present with a mutation in either the COL1A1 or COL1A2 genes.
Aneuploidies (Down Syndrome, Turner Syndrome, Klinefelter Syndrome)
- Characterized by easily recognizable dysmorphic features and/or mental retardation with severity dependent upon degree of chromosomal deletions or duplications.
Case Study[edit | edit source]
Ehlers-Danlos_Syndrome_Case_Study
Case Reports[edit | edit source]
- Erez Y, Ezra Y, Rojansky N. Ehlers-Danlos Syndrome Type IV in Pregnancy. Fetal Diagnosis and Therapy. 2008; 23: 7-9. http://proquest.umi.com.libproxy.bellarmine.edu/pqdweb?did=1400834311&sid=4&Fmt=6&clientId=1870&RQT=309&VName=PQD
- Hollands JK, Santarius T, Kirkpatrick PJ, Higgins IN. Treatment of a direct carotid-cavernous fistula in a patient with type IV Ehlers-Danlos syndrome: a novel approach. Neuroradiology. 2006; 48: 491-494. http://proquest.umi.com.libproxy.bellarmine.edu/pqdweb?did=1073254911&sid=13&Fmt=6&clientId=1870&RQT=309&VName=PQD
- Menawat AS, Panwar RB, Singh HH, Kochar DK, Sulemani AA, Saksena HC. Ehlers-Danlos Syndrome (A Case Report). Journal of Postgraduate Medicine. 1980; 26(2): 142-144. http://www.jpgmonline.com/article.asp?issn=0022-3859;year=1980;volume=26;issue=2;spage=142;epage=4;aulast=Menawat
- Russek LN. Examination and Treatment of a Patient with Hypermobility Syndrome. Physical Therapy. 2000; 80(4): 386-398. http://ptjournal.apta.org/cgi/content/full/80/4/386?hits=10&FIRSTINDEX=0&FULLTEXT=ehlers+danlos&SEARCHID=1&gca=ptjournal%3B80%2F4%2F386&sendit=Get+All+Checked+Abstract%28s%29&
- Sakala EP. Ehlers-Danlos Syndrome Type III and Pregnancy - A Case Report. Journal of Reproductive Medicine. 1991; 36(8): 622-24. http://www.ednf.org/index.php?option=com_content&task=view&id=1492&Itemid=88888988
- Simmonds JV, Keer RJ. 2008. Hypermobility and the hypermobility syndrome. Masterclass. Illustrated via case studies, part II. Man Ther 13:e1–e11.[23]
Resources
[edit | edit source]
Ehlers-Danlos National Foundation: www.ednf.org
MayoClinic: www.mayoclinic.com
Medline Plus: http://www.nlm.nih.gov/medlineplus/
National Institute of Arthritis and Musculoskeletal and Skin Diseases: http://www.niams.nih.gov/
University of Washington, Dept. of Orthopaedics and Sports Medicine: http://www.orthop.washington.edu/
Ehlers-Danlos Syndrome Network C.A.R.E.S.: http://www.ehlersdanlosnetwork.org/
Arthritis Foundation: http://www.arthritis.org/
References[edit | edit source]
see adding references tutorial.
- ↑ Pessler S, Sherry DD. Ehlers Danlos Syndrome. The Merck Manual of Diagnosis and Therapy. http://www.merck.com/mmpe/sec19/ch284/ch284c.html?qt=ehlers%20danlos&alt=sh (Accessed Feb 12, 2010).
- ↑ 2.00 2.01 2.02 2.03 2.04 2.05 2.06 2.07 2.08 2.09 2.10 2.11 2.12 2.13 2.14 2.15 2.16 2.17 2.18 2.19 Steiner RD. Ehlers-Danlos Syndrome. http://emedicine.medscape.com/article/943567-overview (Accessed Feb 15, 2010).
- ↑ 3.0 3.1 3.2 Castori M, Morlino S, Celletti C, Celli M, Morrone A, Colombi M, Camerota F, Grammatico P. Management of pain and fatigue in the joint hypermobility syndrome (a.k.a. Ehlers–Danlos syndrome, hypermobility type): Principles and proposal for a multidisciplinary approach. Am J Med Genet Part A, 2012;158A(8):2055–2070.
- ↑ 4.00 4.01 4.02 4.03 4.04 4.05 4.06 4.07 4.08 4.09 4.10 4.11 4.12 4.13 4.14 4.15 4.16 4.17 4.18 Levy HP. Ehlers-Danlos Syndrome, Hypermobility Type. Gene Reviews. http://www.ncbi.nlm.nih.gov/bookshelf/br.fcgi?book=gene&part=eds3 (Accessed Feb 16, 2010).
- ↑ 5.0 5.1 5.2 5.3 Wenstrup R, Paepe AD. Ehlers-Danlos Syndrome, Classic Type. Gene Reviews. http://www.ncbi.nlm.nih.gov/bookshelf/br.fcgi?book=gene&part=eds (Accessed Mar 3, 2010).
- ↑ 6.0 6.1 6.2 6.3 Yeowell HN, Steinman B. Ehlers-Danlos Syndrome, Kyphoscoliotic Form. Gene Reviews. http://www.ncbi.nlm.nih.gov/bookshelf/br.fcgi?book=gene&part=eds6 (Accessed Mar 3, 2010).
- ↑ 7.0 7.1 7.2 7.3 Pepin MG, Myers PH. Ehlers-Danlos Syndrome, Vascular Type. Gene Reviews. http://www.ncbi.nlm.nih.gov/bookshelf/br.fcgi?book=gene&part=eds4 (Accessed Mar 3, 2010).
- ↑ Ehlers-Danlos syndrome. Genetics Home Reference. 2017. Accessed March 2017. Available from: https://ghr.nlm.nih.gov/condition/ehlers-danlos-syndrome#statisticshttps://ghr.nlm.nih.gov/condition/ehlers-danlos-syndrome#statistics
- ↑ Voermans N, Knoop H, van de Kamp N, Hamel B, Bleijenberg G, van Engelen B. Fatigue Is a Frequent and Clinically Relevant Problem in Ehlers-Danlos Syndrome. 2010;30(3):267-274
- ↑ 10.0 10.1 10.2 10.3 10.4 10.5 Woinarosky N et al. The evidence-based rationale for physical therapy treatment of children, adolescents, and adults diagnosed with joint hypermobility syndrome/hypermobile Ehlers Danlos syndrome. American Journal of Medical Genetics Part C: Seminars in Medical Genetics, 2017;175(1):158-167.
- ↑ 11.0 11.1 Rombaut L, Scheper M, De Wandele I, De Vries J, Meeus M, Malfait F, Engelbert R, Calders P. Chronic pain in patients with the hypermobility type ofEhlers-Danlos syndrome: evidence for generalized hyperalgesia. Clin Rheumatol.fckLR2014 Feb 4
- ↑ 12.0 12.1 12.2 12.3 12.4 12.5 12.6 Tofts LJ, Elliott EJ, Munns C, Pacey V, Sillence DO. The Differential diagnosis of Children with Joint Hypermobility: A Review of the Literature. Pediatric Rheumatology 2009;7:1
- ↑ P.H. Byers, M.L. Murray. Heritable collagen disorders: the paradigm of the Ehlers-Danlos syndrome. J Invest Dermatol, 2012;132(3): E6–E11
- ↑ Liu X, Wu H, Byrne M, Krane S, Jaenisch R. Type III collagen is crucial for collagen I fibrillogenesis and for normal cardiovascular development. Proceedings of the National Academy of Sciences.1997;94(5):1852-1856.
- ↑ Bathen T, Hångmann AB, Hoff M, Andersen LØ, Rand-Hendriksen S. 2013. Multidisciplinary treatment of disability in Ehlers–Danlos syndrome hypermobility type/hypermobility syndrome: A pilot study using a combination of physical and cognitive-behavioral therapy on 12 women. Am J Med Genet Part A. 2013; 161A(12):3005–3011.
- ↑ Bennett Bremner. Spiral Thigh Brace for Ehlers Danlos Syndrome. Available from: http://www.youtube.com/watch?v=LWNiI-YyfE0 [last accessed 27/09/13]
- ↑ caringmedical. Prolotherapy for Hypermobility and Ehlers-Danlos. Available from: http://www.youtube.com/watch?v=6iC7s3sMujc [last accessed 27/09/13]
- ↑ Yeowell HN, Steinmann B. Ehlers-Danlos syndrome, kyphoscoliotic form. NCBI, 2013. Available from: https://www.ncbi.nlm.nih.gov/books/NBK1462/?report=printable
- ↑ Ferrell W, Tennant N, Sturrock R, Ashton L, Creed G, Brydson G et al. Amelioration of symptoms by enhancement of proprioception in patients with joint hypermobility syndrome. Arthritis & Rheumatism. 2004;50(10):3323-3328.
- ↑ Engelbert RH, Juul-Kristensen B, Pacey V, de Wandele I, Smeenk S, Woinarosky N, Sabo S, Scheper MC, Russek L, Simmonds JV. 2017. The evidence-based rationale for physical therapy treatment of children, adolescents, and adults diagnosed with joint hypermobility syndrome/hypermobile Ehlers Danlos syndrome. Am J Med Genet Part C Semin Med Genet 175C:158–167.
- ↑ Russek LN. Examination and Treatment of a Patient with Hypermobility Syndrome. Physical Therapy 2000;80(4):386-398.
- ↑ Russek LN. Hypermobility Syndrome. Physical Therapy 1999;79(6):591-599.
- ↑ Simmonds JV, Keer RJ. 2008. Hypermobility and the hypermobility syndrome. Masterclass. Illustrated via case studies, part II. Man Ther 13:e1–e11.