Ankle and Foot Arthropathies
Original Editors - Ward Willaert
Top Contributors - Anouck Leo, Admin, Arturo Quiroz Marnef, Evelien De Wolf, Scott Cornish, Kim Jackson, Lucinda hampton, Rachael Lowe, Ward Willaert, Gayatri Jadav Upadhyay, Yuli Borremans, 127.0.0.1, Maxim de Clippele, Mariam Hashem, Cath Young, Fitim Cami, Veekhoven Laura, Jan De Backer, Sai Kripa and Aminat Abolade
Description [edit | edit source]
Arthropathy[edit | edit source]
An arthropathy is a disease which affects a joint. Although the terms arthropathy and arthritis have very similar meanings, the former is traditionally used to describe the following conditions:
- Reactive arthropathy occurs as a reaction against an infection site elsewhere in the body.[1]
- Enteropathic arthropathy is an arthropathy in association with, or as a reaction to, an enteric (usually colonic) inflammatory condition.[2]
- Crystal arthropathy is characterised by accumulation of tiny crystals in one or more joints.[3]
- Neuropathic arthropathy is a joint disease caused by diminished proprioceptive sensation, with gradual destruction of the joint by repeated subliminal injury.
- Diabetic arthropathy is a neuropathic arthropathy occurring in diabetic patients. [4]
An arthropathy can be degenerative, such as osteoarthritis, or it can be associated with an inflammation, rheumatoid arthritis for example. A joint disease can also occur following trauma.
Although an arthropathy is distinctly less common in the ankle than in the hip and knee, it is an equally disabling condition.[5]
Arthropathy is a blanket term covering numerous conditions, some specific conditions are discussed in detailed below:
Osteoarthritis[edit | edit source]
Osteoarthritic diseases are a result of both mechanical and biological events that destabilise the normal coupling of degradation and synthesis of articular cartilage chondrocytes, extracellular matrix and subchondral bone. Although they may be initiated by multiple factors, including genetic, developmental, metabolic, and traumatic, OA diseases involve all the tissues of the diathrodial joint.
Ultimately, OA diseases are manifested by morphologic, biochemical, molecular and biomechanical changes of both cells and matrix which can lead to a softening, fibrillation, ulceration, loss of articular cartilage, sclerosis and eburnation of subchondral bone, osteophytes, and subchondral cysts.[6]
Rheumatoid arthritis[edit | edit source]
Studies have categorised rheumatoid foot problems into; forefoot, midfoot and hindfoot pathologies.[7]Most of the studies have focused on forefoot and hindfoot pathologies with a reduced number of studies concentrating on the midfoot RA.[8] Rheumatoid arthritis is a multi-systemic and chronic progressive inflammatory disease. The joints become swollen, tender and painful which can lead to severe disability. [8][9][10][11][12]
Haemophilic arthropathy[edit | edit source]
Haemophilic arthropathy causes a high level of morbidity in patients with severe haemophilia. The degenerative changes that occur in the joints of these patients are usually progressive and result from recurrent bleeding in ‘target’ joints. The ankle is one of the most frequent areas of pain in haemophilic patients. [13]
Diabetic foot arthropathy[edit | edit source]
Charcot neuropathic osteoarthropathy of the foot is a devastating neuropathic complication of diabetes. [14][15][16][17][18][19]
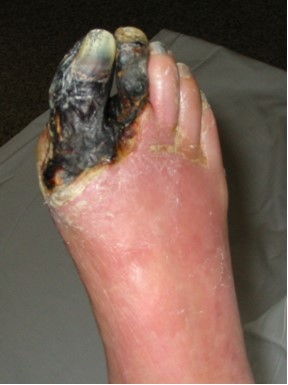
Charcot foot is a progressive and degenerative arthropathy of a single or multiple joints that ultimately leads to destruction of normal foot architecture and collapse of the arch. [16] It also frequently leads to foot ulceration, gangrene and foot amputation. [17][18][19]
The current accepted origin theory of Charcot neuropathic osteoarthropathy states that an unregulated inflammatory process is triggered in patients with peripheral neuropathy. The inflammatory process eventually stimulates the maturation of osteoclasts from osteoclast precursor cells. [17][19]
Gout[edit | edit source]
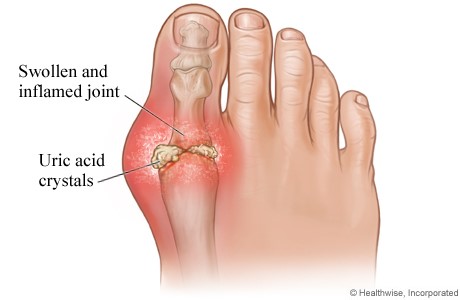
Gout is a crystal-induced arthritis, in which monosodium urate (MSU) crystals precipitate within joints and soft tissues and elicit a highly inflammatory but localised response. The susceptibility to form MSU crystals is a consequence of excessive blood levels of soluble urate, one of the final products of the metabolic breakdown of purine nucleotides. Hyperuricemia is typically defined as occurring above the saturation point of MSU, at which point the risk of crystallisation increases. Using this definition, hyperuricemia occurs at serum urate levels >6.8 mg/dL [20].
Psoriatic arthritis[edit | edit source]
Psoriatic arthritis is an inflammatory arthritis which affects the skin and musculoskeletal system. [21][22] If not diagnosed early and treated effectively it can result in joint deformity and disability. [21]
Psoriatic arthritis is a chronic condition which can cause considerable disability and pain if not recognized and treated properly. Approximately 15% of patients affected by psoriasis will develop associated joint disease. It was first recognized in 1964 and is now considered part of the spondyloarthropathy group of diseases. [21]
Reactive arthritis[edit | edit source]
Reactive arthritis (ReA) is an infectious disease which may be initiated by several microbes in genetically susceptible hosts. [23] Reactive arthritis is one of the types known to primarily affect young men. Because it can be a complication of sexually transmitted infections.
ReA is classified as a type of spondyloarthritis. This group of joint diseases features mono- or oligoarthritis, often associated with extra-articular inflammatory manifestations involving the musculoskeletal, ophthalmologic, dermatologic, and genitourinary systems. These were previously referred to as seronegative spondyloarthritides because the rheumatoid factor is usually negative. [24]
Clinically Relevant Anatomy[edit | edit source]
More information about the anatomy of the ankle and foot can be found on the physiopedia page “Biomechanics of Foot and Ankle” and “Ankle Joint”.
Epidemiology /Etiology[edit | edit source]
Osteoarthritis[edit | edit source]
Approximately 1% of the world’s adult population is affected by ankle OA, which results in pain, dysfunction, and impaired mobility. The mental and physical disability associated with end-stage ankle OA is at least as severe as that associated with end-stage hip OA. Numerous clinical and epidemiologic studies have identified previous trauma as the most common origin of ankle OA. 79.5% of patients with ankle OA had a verified history of 1 or more joint injuries. [13]
Rheumatoid arthritis[edit | edit source]
The prevalence of foot pain in patients with RA has been reported in varying numbers within the published literature. The prevalence of foot pain depends on the stage of the disease. Ranging from 60 to 94 percent at some stage of the disease. [10][11] In the early stages of RA the prevalence is lower: only 16 percent in one study and 32 in another study. [10]
Haemophilic arthropathy[edit | edit source]
It has been demonstrated that recurrent haemorrhages as occurred in haemophilia result in severe joint destruction. Many authors have reported changes in the synovial membrane and cartilage in chronic haemarthrosis. Repeated episodes of intra-articular bleeding cause damage to the joint, leading to deformity.
Several joint disorders, degenerative ones, such as osteoarthritis, inflammation-mediated ones, such as rheumatoid arthritis, and blood-induced ones, such as haemophilic arthropathy, result in cartilage damage and changes in synovial tissue. [25]
Diabetic foot arthropathy[edit | edit source]
Diabetes affects approximately 387 million people worldwide. Together with neuropathy, diabetes mellitus, is currently considered the main cause of Charcot neuropathic osteoarthropathy. The disease goes often undiagnosed among patients suffering of diabetes. The prevalence depends on the way of examination: changes diagnosed by X-ray and corresponding with Charcot neuropathic osteoarthropathy are detected in up to 29% of diabetics. Whereas when MRI is used as a diagnostic method the detection rate rises to 75%.[19]
Gout[edit | edit source]
The global burden of gout is substantial and seems to be increasing in many parts of the world over the past 50 years. The distribution of gout is uneven across the globe, with prevalence being highest in Pacific countries. Developed countries tend to have a higher burden of gout than developing countries, and seem to have increasing prevalence and incidence of the disease. [26]
In various geographic regions, men were more likely to report gout than women [27]. Less than 10% of the cases occur in women. Most women with gout are 15 years or more postmenopausal. This arthropathy is rare in children. [28]
The results of several studies suggest that environmental, racial, and hereditary differences may influence the development of gout. For example, Tokelauan migrants to New Zealand have a greater prevalence of gout than nonmigrants, and certain populations, such as the Maori, have a greater frequency of gout than other New Zealand populations. [27]
Some ethnic groups are particularly susceptible to gout, supporting the importance of genetic predisposition. [26]
Hyperuricemia is the most important risk factor for gout [29] and is affected by both genetic factors and environmental factors [30]. Factors that increase serum urate levels include hypertension, thiazide diuretic intake, obesity, alcohol use, and a high animal protein diet [31].
Socioeconomic and dietary factors, as well as comorbidities and medications that can influence uric acid levels and/or facilitate MSU crystal formation, are also important in determining the risk of developing clinically evident gout. [26]
The risk of gout is lower in men who are more physically active, maintain ideal body weight, and consume diets enriched in fruit and limited in meat and alcohol. [32]
Psoriatic arthritis[edit | edit source]
In comparison to most other rheumatic disorders genetic predisposition plays a major role in the development of Psoriatic arthritis. [33] Psoriasis is known to affect approximately 2% to 3% of the general population, and the prevalence of Psoriatic arthritis in psoriasis patients is between 6% and 39%. It is possible that the condition remains generally underdiagnosed, related to lack of awareness by both the patient and physician. [34]
It is still not clear what exact mechanism lies behind the development of psoriatic arthritis. It is thought to be multifactorial and secondary to environmental, genetic, and immunological factors. When compared with other inflammatory rheumatic conditions, psoriasis and psoriatic arthritis are strongly heritable. [21]
Reactive arthritis[edit | edit source]
Data indicates that approximately 50% of reactive arthritis and undifferentiated oligoarthritis cases can be attributed to a specific pathogen by a combination of culture and serology. The predominant organisms are Chlamydia, Salmonella, Shigella, Yersinia and Campylobactor species. The annual incidence of ReA was found to be 28/100.000 individuals in one study. This may exceed that of rheumatoid arthritis. [35]
Characteristics/Clinical Presentation
[edit | edit source]
The characteristics and clinical presentation of ankle arthropathies such as different forms of arthritis can be described as followed:
- Ankle pain
- Stiffness
- Swelling
- Limited range of motion (ROM)
Pain mostly gets worse by activities such as standing, walking or running.
We can also speak of the so called “Start-up pain” such as when a patient has pain and stiffness in the ankle after sleeping or sitting in one spot for a while is also a common complaint.
When this happens/occurs it often takes the patient a few minutes (or longer) to “warm-up” the ankle. The ankle will tend to swell more as the day progresses particularly if there is increasing activity (patient is still doing sport activities, work activities, …).
Most of the time pain is experienced throughout the ankle although it may be more noticeable in the front of the ankle if large bones spurs have formed. When there has been damage to the joint ankle, it’s often seen that arthritis will occur. Cartilage that normally covers the bones of the ankle joint can be lost leading to an ankle arthropathy.
Osteoarthritis[edit | edit source]
There are four degrees of severity in osteoarthritis:
- Degree I: normal joint with a minimal osteophyte.
- Degree II: osteophytose on two points with minimal subchondral sclerosis, proper joint space and no deformity.
- Degree III: moderate osteophytose, early deformity of the bone endings and a joint space which narrows.
- Degree IV: large osteophytes, deformity of bone endings, narrowing joint space, sclerosis and cysts [36]
Rheumatoid arthritis[edit | edit source]
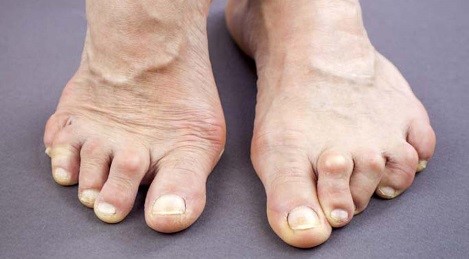
Hallux valgus, splaying of forefoot and pes planus are several of the most typical foot deformities in RA. [8][11] The foot problems caused by RA can lead to mobility problems [12][37], slower gait speed and decrease in toe strength. [10]
A systematic review and meta-analysis by Carroll et al. [38] showed that the majority of the evaluated studies reported gait adaptations in patients with RA. They also have a greater risk of falling than other (elder) people. [11]
Fatigue is a frequent symptom in RA, even among patients who display low and moderate levels of disease activity. As much as 40% of the patients with RA may be severely fatigued. [39]
Haemophilic arthropathy[edit | edit source]
Haemophilic arthropathy refers to permanent joint disease occurring in haemophilia sufferers as a long-term consequence of repeated haemarthrosis. Early in an acute joint bleed, patients may report sensations of tingling and warmth, followed by pain, swelling and decreased motion. The pathogenesis of the progression from recurrent haemarthrosis to arthropathy is incompletely understood, but is characterized by inflammatory synovitis and cartilage destruction. [40]
Haemophilic arthropathy, which shares some clinical and biological injury characteristics with rheumatoid arthritis, is characterized by two main features: chronic proliferative synovitis and cartilage destruction. It is the consequence of repeated extravasation of blood into joint cavities, but its exact pathogenesis, particularly with regard to early changes in the joint, is still incompletely understood. [41]
Over the long-term, repeated episodes of haemarthrosis may cause irreversible damage to the joint, leading to haemophilic arthropathy, a polyarticular disease characterized by joint stiffness, chronic pain and a severely limited range of motion. In the most severe cases, the bones become fused, resulting in a completely disabled and misshapen joint. [41]
Diabetic foot arthropathy[edit | edit source]
The Eichenholz classification describes the evolution of the condition through time. [19][42]
- Stage 0: Hot foot, usually somewhat painful normal radiographic findings; MRI will show bone oedema and stress fractures
- Stage 1: Fragmentation, bone resorption, dislocations, fractures
- Stage 2: Coalescence, sclerosis, fracture healing, debris resorption
- Stage 3: Bone remodelling
Gout[edit | edit source]
There are four phases of gout. They include asymptomatic hyperuricemia, acute gouty arthritis, intercritical gout and chronic tophaceous gout. [43]
- Asymptomatic hyperuricemia: Serum urate of more than 7 mg/dl but no symptoms are present. [28]
- Acute gouty arthritis: Exquisite joint pain, occurring suddenly at night, commonly in the first metatarsophalangeal joint. Besides local, intense pain of quick onset, erythema, warmth and extreme tenderness and hypersensitivity are typically present. Chills, fever and tachycardia may accompany the joint complains. [28]
- Intercritical gout: Asymptomatic phase. [28]
- Tophaceous gout: Gouty attacks return suddenly with increasing frequency and often in different joints. Characteristics are joint damage, functional loss, disability, deposits of monosodium urate crystals in soft tissue (= tophi) and bone abnormalities. [28]
Psoriatic arthritis[edit | edit source]
Symptoms that are typical for psoriatic arthritis include inflammation in the Achilles tendon (at the back of the heel) or the Plantar fascia (bottom of the feet), and dactylitis (sausage-like swelling of the fingers or toes). [44]
Pain, swelling, or stiffness in one or more joints is commonly present in psoriatic arthritis. [33] Two features which are hallmarks of psoriatic arthritis are the presence of dactylitis and enthesitis. [21]
Reactive arthritis[edit | edit source]
Reactive arthritis characteristically involves the joints of the lower extremities in an asymmetric, oligoarticular pattern. A dactylitis (‘sausage digit’) pattern in the feet is typical of reactive arthritis. Enthesopathy (inflammation at the sites of insertion of tendons and ligaments into bone) is often found in reactive arthritis. [35]
Differential Diagnosis[edit | edit source]
Intra-articular pathologic lesions must be distinguished from surrounding joint tendinitis and bursitis. This can be achieved with diagnostic testing such as magnetic resonance imaging or with injection of local anesthetic.
Avascular necrosis must be considered in cases in which sclerosis of the talar dome is present. Patients may have a history of talar neck fracture, steroid or alcohol usage, or nonspecific injuries. Avascular necrosis of the talus can result in progressive segmental collapse and an increasing amount of particulate matter into the joint.
Osteoarthritis-Rheumatoid arthritis[edit | edit source]
Primary osteoarthritis is a diagnosis of exclusion.
Post traumatic osteoarthritisis the most common form of ankle arthritis. Post-traumatic disease can be present after intra-articular fractures or improper joint biomechanics after extra-articular fractures. Frequently, deformity is present in the joint. The extent of bone loss after trauma and joint space collapse can be assessed with weightbearing radiographs and CT scans.
Osteoarthritis is best differentiated from rheumatoid arthritis by a careful history and examination. Two factors to distinguish the two disorders: the absence of systemic inflammatory signs and symptoms, onset in later life, and the pattern of joint involvement.
Systematic inflammatory diseases such as rheumatoid arthritis should be excluded prior to considering operative intervention. Ankle arthritis can be effectively treated with a medical regimen prior to considering surgical intervention, particularly during a flare of the disease. The majority of patients with rheumatoid arthritis test positive for rheumatoid factor. In addition, the diagnosis of rheumatoid arthritis requires the presence of certain other symptoms: morning stiffness, multiple joint swelling, rheumatoid nodules, and joint erosion on radiographs.
Patients with absence of rheumatoid factor in the serum, but manifestations of inflammatory arthritis are classified as having seronegative arthropathy. The four major disorders include ankylosis spondylitis, psoriatic arthritis, Reiter’s syndrome, and inflammatory bowel arthritides.
Metabolic and infectious causes of arthritis must be considered as well. This can include gonococcal disease, Lyme disease, and gouty uricemia. Patients should be questioned about possible exposure to disease sources for sexually transmitted diseases and insect bites.
Haemophilic arthropathy[edit | edit source]
H(a)emophilic arthropathy occurs by people who have haemophily, this is a desease which unables the blood from bleeding. When these bleedings occur within the joint it causes multiple defects to the joint, this is the result of a number of mechanisms affecting the synovial lining which becomes progressively fibrotic and the hyaline cartilage which disintegrates and is eventually lost. Mechanical and chemical processes cause degeneration of cells but enzymatic processes appear to be primairily responsible for the degradation of the matrix of the articular cartilage.
Charcot osteoarthropathy or pedal neuropathic joint disease is a condition associated with peripheral neuropathy , it is a progressive deterioration of weight-bearing joints, usually in the foot or ankle, and is characterised in its early stages by acute inflammation that leads to bone and joint fracture, dislocation, instability and Gross deformaties. in patients with diabetes, Charcot osteoarthropathy is associated with a longstanding duration of diabetes and peripheral neuropathy. In the early stages of Charcot osteoarthropathy, the patient presents with a warm, erythematous and oedematous foot with or without associated pain or reported previous injury and can clinically mimic cellulitis or gout.. It can lead to gross structural deformities of the foot and ankle, and subsequent skin ulceration and lower limb amputation from soft tissue or bony infection. The Charcot foot occurs most often in patients with diabetic neuropathy; other predisposing conditions include alcoholic neuropathy, sensory loss caused by cerebral palsy or leprosy, and congenital insensitivity to pain. However, it is often unrecognised, with deleterious consequences..
The differentiation between osteomyelitis and diabetic foot arthropathy is crucial but can be difficult when both acute inflammation and bone chances are present. [15][19]
Gout[edit | edit source]
Gout may masquerade as septic arthritis, rheumatoid arthritis or neoplasm. [28]
Pseudogout often resembles gout and, like gout, is caused by the formation of crystals in the joints. Instead of being composed of uric acid, as true gout crystals are, the crystals in pseudogout are composed of a salt called calcium pyrophosophate dehydrate (CPPD). The condition is also called CPPD disease. [45]
Diagnostic Procedures[edit | edit source]
Osteoarthritis[edit | edit source]
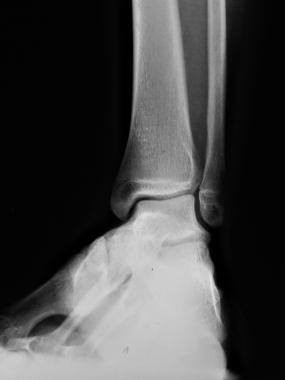
The diagnosis of osteoarthritic ankle joint starts with clinical assessment, and includes assessment of alignment and stability and measurement of range of motion. Different radiographic modalities may help to recognize and analyse the underlying reasons for ankle OA. Only weight-bearing radiographs of the foot and ankle should be performed. Additional imaging modalities such as MRI and SPECT-CT may help to evaluate the extent of degenerative changes and their biological activities. [13]
Rheumatoid arthritis[edit | edit source]
Different imaging techniques, e.g. MRI, CT and ultrasonography (US), should help clinicians to detect early or subclinical foot problems, because clinical signs of foot disease in RA are often subtle. [8][46]
When detecting joint inflammation ultrasonography and MRI have shown to be superior to clinical examination. [47] Sonography is being used more and more and has been found effective for the detection of erosions in patients with RA. Ultrasonography detected 6.5-fold more erosions in early disease than radiography. [46] Because US is easily available and less expensive than MRI it can be recommended as the first imaging method after plain radiography. [47]
Haemophilic arthropathy[edit | edit source]
Radiography remains the workforce horse in the diagnosis and follow-up of haemophilic arthropathy. The radiographical findings in arthropathy follow an expected sequence of events and are overall similar in different joints. Magnetic resonance imaging (MRI) has advantages over radiography based on its capability of visualizing soft tissue and cartilage changes in haemophilic joints. The recent development and standardization of MRI scoring systems for measuring soft tissue and cartilage abnormalities may enable the comparison of pathological joint findings in clinical trials conducted at different institutions across the world [48]
Diabetic foot arthropathy[edit | edit source]
The diagnosis is based on patient’s history, clinical examination, and imaging methods. As a result of their lowered perception of pain, patients are quite often not aware of any injury.[19] Local inflammation is the main symptom which can lead to the diagnosis being suspected. [15]
In Charcot feet arthropathies it is very important that the disease is diagnosed quickly, because a delay can lead to worsening structural damage or even limb loss.[15][49][50][51] Unfortunately the diagnosis is often missed at first presentation. A possible reason for the missed diagnosis is that Charcot feet are not emphasized in medical training. The result is that it is difficult to advocate the right choice of approach due to low evidence based information. [15]
Acute Charcot activity can be diagnosed if the temperature of the affected foot is 2°C or more than the contralateral unaffected foot. [51][19]
Gout[edit | edit source]
Gout is ideally diagnosed through identification of characteristic negatively birefringent crystals under polarized light microscopy in fluid aspirated from end-organ deposits, typically from a joint [52]. However, fewer than 10% of patients with gout see a rheumatologist, and most cases of gout are diagnosed in the primary care setting based on signs, symptoms, and serum uric acid level [53].
Psoriatic arthritis[edit | edit source]
A diagnostic test for psoriatic arthritis does not exist unlike in RA which is cyclic citrullinated peptide and rheumatoid factor positive. As in other inflammatory conditions, markers such as erythrocyte sedimentation rate and C-reactive protein can be raised in psoriatic arthritis. [21]
Scoring systems have been developed to try and identify psoriatic arthritis at an early stage and criteria have been developed to aid in classification of the disease from the other SPAs and inflammatory arthritides. Not only are they useful for identifying psoriatic arthritis earlier, they can also help identify cases of psoriatic arthritis which do not present in the typical manner. Some criteria include psoriatic arthritis with the SPA group. The classification for psoriatic arthritis (CASPAR) criteria was developed specifically for psoriatic arthritis. It has good sensitivity and specificity for those presenting with disease of <2 years’ duration. Although primarily used for classification, it can be used for diagnostic purposes. [21]
Further imaging such as magnetic resonance imaging (MRI) can help to identify soft tissue involvement in further detail, particularly when a patient is suffering from enthesitis. Ultrasound has also become a useful tool in the investigation of arthritis; it can help to identify bony erosions in those patients where synovitis or dactylitis is not always evident clinically. Studies have shown that ultrasound scan and MRI are more sensitive for detecting inflammation than plain radiographs
in psoriatic arthritis. [21]
Outcome Measures[edit | edit source]
(also see "Outcome Measures")
Osteoarthritis[edit | edit source]
The Ankle Osteoarthritis Scale (two subscales: pain and disability) [54] is a reliable and valid self-assessment instrument that specifically measures patient symptoms and disabilities related to ankle arthritis.[55]
More outcome measures of ankle osteoarthritis can be found on the physiopedia page "Ankle Osteoarthritis Arthritis”
Rheumatoid arthritis[edit | edit source]
American College of Rheumatology (ACR) response criteria for RA. [56]
The ACR20 response criteria require a 20% improvement in both tender and swollen joint counts, and a 20% improvement in 3 of 5 items: patient global assessment (visual analog scale, VAS), physician global assessment (VAS), patient pain score (VAS), Health Assessment Questionnaire (HAQ), and either erythrocyte sedimentation rate or C-reactive protein (CRP). [56]
Haemophilic arthropathy[edit | edit source]
Visualization of bone or cartilage damage in index joints on MRI can be used as outcome measure
Tentative haemophilic arthropathy scales based on MRI findings have been developed in the last decade. In 2005, the International Prophylaxis Study Group (IPSG) presented a preliminary comprehensive scoring scheme that combined the pioneer Denver and European MRI scores. The use of such scales should result in a more consistent assessment of haemophilic joints and should facilitate the development of more targeted treatment to prevent or delay further destructive osteoarticular changes.[48]
Diabetic foot arthropathy[edit | edit source]
No research found.
Gout[edit | edit source]
Many different instruments can be used to assess the acute gout core domains. Pain VAS and 5-point Likert scales, 4-point Likert scales of index joint swelling and tenderness and 5-point PGART instruments meet the criteria for the OMERACT filter. [57]
Psoriatic arthritis[edit | edit source]
The Psoriatic Arthritis Response Criteria (PsARC) is recommended in the assessment and monitoring of PsA. It consists of four components: assessment of joint tenderness and swelling utilizing 68/66 joint counts respectively, the patient’s opinion of their global health and the physician’s global assessment. [56][58]
The ACR20 response criteria require a 20% improvement in both tender and swollen joint counts, and a 20% improvement in 3 of 5 items: patient global assessment (visual analog scale, VAS), physician global assessment (VAS), patient pain score (VAS), Health Assessment Questionnaire (HAQ), and either erythrocyte sedimentation rate or C-reactive protein (CRP). For some PsAstudies the joint count was increased to 78 to include distal interphalangeal (DIP) joints of the feet. To achieve an ACR50 or ACR70 response, the same guidelines apply but the level of response is 50% or 70% improvement, respectively. [56]
Reactive arthritis[edit | edit source]
The outcome measures of reactive arthritis can be found on the physiopedia page "Reactive Arthritis”
Examination[edit | edit source]
Osteoarthritis[edit | edit source]
The examination of osteoarthritis can be found on the physiopedia page "Ankle Osteoarthritis”
Rheumatoid arthritis[edit | edit source]
The examination of rheumatoid arthritis can be found on the Physiopedia page "Rheumatoid Arthritis”
Medical Management
[edit | edit source]
Osteoarthritis[edit | edit source]
There is no cure of osteoarthritis. There are several treatments we can subdivide in pharmacologically, non- pharmacologically and surgical. The choice of treatment of ankle and foot osteoarthritis(OA) depends on the severity of the disease. The goal of managing OA in foot and ankle includes the control of pain, improvement in function and quality of life. A number of different aspects like discomfort, comorbidity and radiologic damage need to be considered[59]
Pharmacological treatments
|
Simple painkillers like paracetamol or acetaminophen are most of the time enough to have a significant relief of symptoms, for patients with mild to moderate pain[59] | |
| NSAID |
|
| Opioids |
When NSAID doesn’t give enough relief of symptoms, opioids are used. These opioids decrease the pain during a long–term treatment which increases sleep and enjoyment of life. Common opioid side effects are present but decrease in duration as the therapy continues[61]. |
|
When simple analgesics have failed, these injections are used in a hospital environment. Most of the time, hyaluronic acid is recommended if there is inadequate response to simple analgesics. Hyaluronic acid Is a natural component of synovial fluid. These gel-like fluid injections help to lubricate the joint and to act as a shock absorber for joint loads[60]. Researched indicated that there is an improvement in pain, but repeated injections in the same joint showed decreased response[59]. Otherwise it remains unclear which dosage schedule should be used and which patients benefit the most from it. Other research showed that there is a relief of pain, but more difficulty in walking on uneven surface, especially when we compare this with exercise therap[62]. |
Surgical treatment
| Osteotomy |
Low tibial osteotomy (a change of alignment of the bone) is indicated for osteoarthritis in the ankle, more specifically for varus-type osteoarthritis in a moderated stage[63]. |
| Arthroscopy |
There was no significant improvement in instability. But there was significant improvement in pain, swelling, stiffness, limp, and activity in the patients postoperatively compared with their preoperative status. But not with every patient. Approximately one third of the patients had no benefit from the arthroscopic intervention[64]. |
| Arthrodesis |
During an arthrodesis operation, the joint becomes fixed. So, after this operation, there is no movement in the joint possible. This method is used in end-stage osteoarthritis, but research shows that arthroplasty gives a better pain relief[65]. |
| Arthroplasty |
This method is, like arthrodesis, a method mostly used in end-stage osteoarthritis. This is a total replacement of the affected joint. This is the most effective of all medical interventions. The pain and disability of end-stage osteoarthritis can be eliminated and restores patients to near normal function[66]. |
Rheumatoid arthritis[edit | edit source]
Pharmacological treatments
| DMARD’s |
|
| Anti-TNF treatment |
The use of anti-TNF therapy on patients suffering from RA have accelerated ulcer healing[67]. Regarding the level of evidence of this article one should be cautious generalizing the results to all the patient suffering from rheumatoid arthritis. The difference between DMARD combination treatments, including or excluding TNF inhibitors, is small. Due to the enormous cost differences, RA guidelines should recommend combination DMARD treatment before initiation of TNF inhibitors[68]. |
| Steroids |
|
Haemophilic arthropathy[edit | edit source]
Pharmacological treatments
| Narcotics |
This is effective bus it leads to dependence[40]. |
| NSAIDs |
Those NSAIDS act by inhibiting cyclooxygenase enzymes, which result in an analgesic and anti-inflammatory effect. |
| Steroid-/ hyaluronic acid-injections |
Both injections decrease the pain[40] |
Surgical treatments
| Synovectomy |
This is an excision or destruction of the friable synovium. This approach is frequently used to reduce pain in patients who experience recurrent haemarthrosis. This can be achieved by direct surgical excision or arthroscopic synovectomy. Another way is to inject a radioactive or chemical agent which causes fibrosis of sclerosis of the synovium. If there are several advanced changes in the joint, a joint arthroplasty may be considered[40]. |
| Joint arthroplasty |
|
Diabetic foot arthropathy[edit | edit source]
A multidisciplinary approach is recommended for the management of Charcot foot involving medical and allied health professionals.[18]
Pharmacological treatments
| Biphosphonates |
There is currently little evidence to support the use of bisphosphonates as part of the routine management of patients with diabetes complicated by acute Charcot neuropathic osteoarthropathy[70][19][42] But some authors found that bisphosphonates may improve the healing of Charcot foot by reducing skin temperature and disease activity of Charcot foot. Bisphosphonates should be used in addition to standard interventions to control the position and shape of the foot[15]. |
| Anti-TNF treatment |
It is plausible to suggest the use of anti-TNF biological therapies for controlling Charcot neuropathic osteoarthropathy based on the excessive bone resorption due to circulating osteoclast precursors and serum levels of TNF-a[16]. |
Surgical treatments
| Surgery |
Surgery should be avoided during the active inflammatory stage because of the perceived risk of wound infection[42].A patient only becomes a candidate for surgical treatment after the failure of conservative management[19]. |
| Magnetic therapy treatment |
|
Gout
Pharmacotherapy
| Urate level |
The goal of pharmacotherapy for gout is to maintain the serum urate level below 50–60 mg/L (300–360 moll/L). When urate levels fall below 50–60 mg/L, the urate crystals start to dissolve [31]. Currently-used urate lowering medications include xanthine-oxidase inhibitors such as allopurinol, febuxostat, as well as available uricosuric agents. However, evidence comparing these agents remains scant. Based on the reported data, febuxostat can play a major role in the treatment of hyperuricaemia and gout. Febuxostat is a suitable pharmacological option for first line treatment of gout, given its established efficacy and safety, documented in a high number of clinical studies and in daily practice[71] |
| NSAIDs |
These are frequently used as first-line therapies for acute gout. However, these agents may have serious side effects such as gastrointestinal toxicity, renal toxicity, or gastrointestinal bleeding[72] |
| Systemic corticosteroids |
Systemic corticosteroids have also exhibited significant efficacy in patients with acute gout; intra-articular corticosteroids are frequently used in patients with monoarticular gout, particularly in patients who cannot receive oral therapy[72]. |
| ACTH (Adrenocorticotropic hormone) |
This is also quite useful in treating acute gout, particularly in those patients with renal and/or gastrointestinal contraindications to other therapies. Synthetic ACTH is effective partly via induction of adrenal glucocorticosteroids, and partly via rapid peripheral suppression of leukocyte activation by melatonin receptor 3 signalling[72] |
| Oral colchicine |
In patients with contraindications or who cannot tolerate NSAIDs or systemic (either oral or parenteral) corticosteroids, oral colchicine is generally the next choice for primary therapy[72] |
Psoriatic arthritis[edit | edit source]
Treatments such as oral disease modifying anti-rheumatic drugs and biologic therapy are effective but have side effects which could limit their use in certain individuals[21]
Pharmacological treatments
| Corticosteroids |
Corticosteroids are used in psoriatic arthritis and in many other inflammatory arthropathies to provide immediate symptom relief. They can rapidly diminish the inflammatory response seen in these conditions, causing a reduction in swelling and stiffness in the joints. They can be given as an intramuscular injection for general widespread relief or directly injected into the affected joint for a more targeted response. Long-term steroid use should be avoided due to the side effect profile which includes diabetes, hypertension, osteoporosis, and immunosuppression. There is also a risk of skin psoriasis flares after intramuscular injections. In most cases steroids are used for short-term relief and occasionally as bridging therapy while establishing the patient on more long-term immunosuppressants[21] |
| Disease‐modifying anti‐rheumatic agents or immunosuppressive drugs |
Disease modifying anti-rheumatic drugs (DMARDs) are used in psoriatic arthritis as in other inflammatory conditions. They are a group of drugs which can help to reduce inflammation as well as to slow disease progression to prevent joint damage, as opposed to nonsteroidal anti-inflammatory drugs and steroids which treat the inflammation but not the underlying cause. However, evidence behind their use in psoriatic arthritis is not as robust as in RA. Use of DMARDs is mostly based on a clinician’s experience rather than evidence. Methotrexate can cause improvement in both the skin and peripheral joints. Sulfasalazine is useful for peripheral arthropathy although only with weak evidence. Cyclosporine has beneficial effects on the skin. Small studies have shown that leflunomide has similar efficacy for use in psoriatic arthritis as compared with RA. The main side effect with DMARDs is their immunosuppression, which can expose the patient to potentially serious infections such as neutropenic sepsis[21] |
| Intra –articular injections: TNF-α blockers | Biologic drugs that are aimed at blocking TNF-α have been effective therapies for such conditions[21] |
Reactive arthritis[edit | edit source]
The treatment of reactive arthritis comprises mainly non-steroidal anti-inflammatory drugs, intra-articular steroid injections, and physical treatment. ([73]-Level of evidence:1B)
Pharmacological treatments
| NSAIDs |
Non‐steroidal anti‐inflammatory drugs (NSAIDs) form the basis of pharmacological therapy. They should be used in proper dosage and not be stopped too early. Young persons are often reluctant to take ‘pain killers’. It should be explained that not only the analgesia but the anti‐inflammatory effect is wanted, and especially the latter requires sufficient dosage for sufficient time. During recovery, insufficient analgesia may lead to limited use of the joints and prolong the rehabilitation. Later on, the patient should not unnecessarily use these drugs[23]. |
|
Corticosteroids are a potent group of drugs to be used in ReA. Intra‐articular application results most often in prompt relief of the joint inflammation. This is of great value for a young, active and employed person. The injection can be repeated a few times, if necessary. However, this treatment is not applicable if many joints are involved. The risk of infectious complications and true septic arthritis as a differential diagnostic possibility must be kept in mind[23]. | |
| Antibiotics |
Antibiotics seem not to be effective in postenteric reactive arthritis. When the patient seeks help for joint inflammation, the initial infection has already passed, and antibiotics are no longer effective[75]. |
|
The value of disease‐modifying anti‐rheumatic agents or immunosuppressive drugs has not been established. The observations reported by the group of Mielants and Veys[76]. indicate that ileocolonoscopy should be performed for patients with ReA more often than is customary now. | |
|
Intra-articular steroid injections can be used as treatment for reactive arthritis[73]. | |
| TNF-α blockers |
In more aggressive cases, or when ReA evolves towards ankylosing spondylitis, TNF-α blockers could represent an effective choice[77]. |
Physical Therapy Management
[edit | edit source]
Osteoarthritis
[edit | edit source]
Non-care management and care management have been tested. Non-care management include resting and relaxing, tough evidence for their effectiveness has not proved conclusive. On the other hand, physical activity and/or exercise is generally adopted and recognised by health professionals and patients. Specific joints can be targeted to improve general mobility, function and the reduce of pain. More intensive exercise can strengthen muscles around the affected joint of the foot. A limited number of OA patients may have increased pain symptoms while exercising. But with an exercise program that has an appropriate target and is adjusted to the individual pace, it should be possible for every OA patient to do some exercise/sports[78].
Non- Pharmacological treatments
| Aerobic exercise | Aerobic exercises include different activities such as walking, cycling, dancing or chair-based exercises. Some guidelines were developed for training parameters in people with osteoarthritis pain.
|
| Range of motion and flexibility exercise | Joint motion and elasticity of periarticular tissues on a regular basis are important for cartilage nutrition and health, protection of joint structures from damaging impact loads and comfort in daily activities. Those exercises are routines of low-intensity, controlled movements that do not increase the pain in foot or ankle[79]. |
Rheumatoid arthritis
[edit | edit source]
Physical therapy
| Exercise therapy | It is essential that patients adhere to their training program even when supervision is withdrawn. Physiotherapists should inform their patients that adherence is crucial because the gains in muscle mass and strength dependent function diminish when the training stops. The damage of the feet (via Larsen score) were increased significantly less in the participants in the exercise group than in the participants of the usual care physical therapy group[62]. Exercise sessions should be consequently performed because analyses showed that the lower the attendance rate at exercise sessions the more the rate of damage increased in the foot joints[62]. The patients with RA who participate in long term high intensity weight bearing exercise classes will develop less radiological joint damage in the feet in comparison with patients participating in usual care physical therapy[62]. The results of the meta-analysis from Baillet et al. Suggests that resistance exercise therapy is safe and offers a clinically significant amelioration of functional capacity and disability. However, they didn’t find better results in decrease in disability and functional capacity amelioration when comparing supervised exercises with home-based exercises. Further RCT’s are required to determine whether supervised exercises are clinically relevant[12]. The maintenance of long-term positive effects gained from resistance exercise has not been extensively evaluated, and remains controversial, yet it is crucial for long-term benefits that after the intervention a structured physical activity program is maintained, because the effects of detraining occur early[12][12]. A 3 year follow up after completing a high-intensity progressive resistance training program Lemmey et al. showed a significant maintenance in adiposity measures benefit and walk test performance. Whereas lean mass and more strength-dependent physical function measures were completely lost[80]. |
Custom-made Foot Orthoses
| (Orthotic) Insoles and specialized shoes | The continuous use of insoles combined with prescribed forefoot rocketed shoes with wide toebox resulted in a significant reduction in foot pain and dysfunction in patients with rheumatoid foot problems. However custom-made insoles showed no differences in terms of anatomical locations of foot pathology[81].
|
| Foot Orthoses | In a systematic review involving 110 patients with Rheumatoid Arthritis Conceição et al. found pain improvements due to foot orthoses. But it had no effect on disability[85] |
Haemophilic arthropathy[edit | edit source]
Physiotherapy is an integral component in haemophilic foot and ankle arthropathy. Physiotherapy can be used when the arthropathy is acute, chronic or after surgery. To prevent the progression of haemophilic arthropathy, regular exercise (30 min at least three time/week) are necessary[40]
The goal of this therapy is to reduce bleeding, pain and improve articular mobility of the ankle[86].
Physical therapy
| Goals of the therapy | -restoration/maintenance of ROM -Muscle strengthening -pain management -improved balance/coordination/ proprioception[40]. |
| Varied therapy | -passive mobilizations -passive/active stretching (specifically: the anterior and posterior tibialis, peroneal, gastrocnemius and soleus muscles) -proprioception exercises -active exercises of strength with Thera- band -joint stabilisation exercise[86]. |
Custom-made Foot Orthoses
| Orthopaedic insoles(OI) and shoes(OS) | The study of Lobet et al. showed that foot orthoses may have beneficial effects on ankle joints in hemophilic arthropathy. OI and OS provided significant pain relief, functional improvement and improved comfort in more than half of patients, with minimal side effects[87]. |
| OI/OS combined with physiotherapy | Two other researchers observed that combined physiotherapy and OI/OS resulted in excellent patient satisfaction scores and significant pain reduction[87]. |
Diabetic foot arthropathy[edit | edit source]
The treatment of Charcot neuroarthropathy is mostly conservative[19]. The first step of treatment in the management goals of Charcot foot are to offload the affected extremity, prevent further collapse and deformity and protect the opposite foot. This can be done by a castor splint immobilization to promote eventual healing of the joint during the active stage of the disease[88][19][42].
Non- Pharmacological treatments
| Total contact cast | The use of a total contact cast in an early stage of Charcot foot is also recommended[89] It should be changed 3 days after initial application and then every week. The period of fixation depends on the reduction in oedema and a drop in skin temperature below 2°C compared to the contralateral leg. The usage of a wheelchair as a preventive means against overloading the other extremity can be considered[19] |
|
CROW Figure 10 |
Alternatively, it is also possible to use Charcot Restraint Orthotic Walker (CROW). In the study of Kalish et al[88] they searched the MEDLINE database to identify articles on diabetic foot problems and found that the use of accommodative footwear is essential for a long-term effective management of Charcot foot. |
Gout[edit | edit source]
The physical therapist should be aware that any patient with a history of gout, hyperuricemia, and/or a septic joint presentation should be referred for medical evaluation before treatment. [28]
During acute exacerbations, the physical therapist should focus on reinforcement of management program and splinting, orthotics, or other assistive devices to protect the affected joint(s). During intercritical phases physical therapists may offer assistance with maintenance of ROM, strength, and function. The physical therapist can also assist the patient in the creation of a suitable exercise routine and keeping their weight under control. [28]
Non- Pharmacological treatments
| Cryotherapy | There is a study that shows that cold applications, also called cryotherapy, may be a useful adjunct to treatment of acute gouty arthritis. The group treated with ice had a significantly greater reduction in pain compared with the control group. Although the clinical improvement was impressive, due to the small sample size they could not show statistically significant improvement[90] |
| Acupuncture | The effectiveness of acupuncture as complementary therapy was reviewed and suggest that it is effective for gouty arthritis patients. Ten RCTs involving 852 gouty arthritis patients were systematically reviewed. Among them six studies of 512 patients reported a significant decrease in uric acid in the treatment group compared with a control group, while two studies of 120 patients reported no significant decrease in uric acid in the treatment group compared with the control group. The remaining four studies of 380 patients reported a significant decrease in visual analogue scale score in the treatment group[91] |
Psoriatic arthritis[edit | edit source]
The physical therapy management of psoriatic arthritis can be found on the Physiopedia page “Psoriatic Arthritis”
Reactive arthritis[edit | edit source]
An exercise regimen that includes regular aerobic activity as well as exercises that promote joint range of motion and muscles strengthening should be utilized. Strengthening should target muscles surrounding the affected joints with the purpose of improving its support system.
You may choose to recommend a short period of non-weight bearing, to decrease the inflammation and limit the pressure of the body on the inflamed joints.
Immobilization and inactivity are discouraged as they can lead to decreased range of motion, contractures, joint stiffness, decreased muscle strength and decreased flexibility, as well as overall decreased cardiovascular fitness, which can cause a cascade effect on other body systems. [92]
Physical therapy
| Goals of the therapy | Goals of treatment should include pain relief, improved activities of daily living, reduce joint swelling, prevention of joint damage and disability. [92] |
| Aerobic exerciseAerobic exercise | Aerobic exercise should include low impact activities, such as swimming, walking, or recumbent bike, depending on the patient's cardiovascular level. [92] |
| Range of motion and flexibility exercise | Stretching and ROM exercises should be completed to prevent muscle atrophy, especially if several joints are involved[24][23] The objective of physiotherapy is to avoid stiffness and deformities and to promote mobility and strength[93][94]. |
Custom-made Foot Orthoses
| Orthopaedic insoles(OI) and shoes(OS) | If enthesitis is present, heel support and orthosis can be considered to decrease pain and thus improve mobility[23][95][24] |
Key Research[edit | edit source]
Osteoarthritis[edit | edit source]
D.M. Reid, C.G. Miller (eds.), Clinical Trials in Rheumatoid Arthritis and Osteoarthritis, Springer-Verlag London Limited 2008, 325p. (Level of evidence: 1A) (Last consulted on 19/11/2016)
Witteveen, Angelique GH, Cheriel J. Hofstad, and Gino MMJ Kerkhoffs. "Hyaluronic acid and other conservative treatment options for osteoarthritis of the ankle." The Cochrane Library (2015). (Level of evidence: 1A) (Last consulted on 19/11/2016)
Roth, Sanford H., et al. "Around-the-clock, controlled-release oxycodone therapy for osteoarthritis-related pain: placebo-controlled trial and long-term evaluation." Archives of Internal Medicine 160.6 (2000): 853-860. (level of evidence: 1B) (last consulted on 19/11/2016)
Karatosun, V., et al. "Intra-articular hyaluronic acid compared to exercise therapy in osteoarthritis of the ankle. A prospective randomized trial with long-term follow-up." Clinical and experimental rheumatology 26.2 (2008): 288. (level of evidence: 1B) (last consulted on 19/11/2016)
Rheumatoid arthritis[edit | edit source]
Conceição, Cristiano Sena da, et al. "Systematic review and meta-analysis of effects of foot orthoses on pain and disability in rheumatoid arthritis patients." Disability and rehabilitation 37.14 (2015): 1209-1213. (Level of Evidence 1A) (last consulted on 02/12/2016.)
Mejjad, Othmane, et al. "Foot orthotics decrease pain but do not improve gait in rheumatoid arthritis patients." Joint Bone Spine 71.6 (2004): 542-545. (Level of Evidence 1A) (last consulted on 04/12/2016.)
Baillet, Athan, et al. "Efficacy of resistance exercises in rheumatoid arthritis: meta-analysis of randomized controlled trials." Rheumatology 51.3 (2012): 519-527. (Level of Evidence 1A) (last consulted on 04/12/2016.)
Rongen‐van Dartel, S. A. A., et al. "Effect of Aerobic Exercise Training on Fatigue in Rheumatoid Arthritis: A Meta‐Analysis." Arthritis care & research67.8 (2015): 1054-1062. A Meta-Analysis, Arthritis Care & Research, Vol. 67, No. 8, August 2015, pp 1054–1062. (Level of Evidence 1A) (last consulted on 04/12/2016.)
Carroll, Matthew, et al. "Gait characteristics associated with the foot and ankle in inflammatory arthritis: a systematic review and meta-analysis." BMC musculoskeletal disorders 16.1 (2015): 1. (Level of Evidence 1A) (last consulted on 04/12/2016.)
Graudal, Niels, et al. "Combination Therapy With and Without Tumor Necrosis Factor Inhibitors in Rheumatoid Arthritis: A Meta‐Analysis of Randomized Trials." Arthritis care & research 67.11 (2015): 1487-1495. (Level of Evidence 1A) (last consulted on 04/12/2016.)
Diabetic foot arthropathy[edit | edit source]
Kalish, Jeffrey, and Allen Hamdan. "Management of diabetic foot problems." Journal of vascular surgery 51.2 (2010): 476-486. (Level of evidence: 1A) (last consulted on 20/11/2016)
Varma, Ajit Kumar. "Charcot neuroarthropathy of the foot and ankle: a review." The journal of foot and ankle surgery 52.6 (2013): 740-749. (Level of evidence: 1A) (last consulted on 05/12/2016)
Smith, Caroline, Saravana Kumar, and Ryan Causby. "The effectiveness of non‐surgical interventions in the treatment of Charcot foot." International Journal of Evidence‐Based Healthcare 5.4 (2007): 437-449. (Level of evidence: 1A) (last consulted on 05/12/2016)
Kucera, Tomas, Haroun Hassan Shaikh, and Pavel Sponer. "Charcot Neuropathic Arthropathy of the Foot: A Literature Review and Single-Center Experience." Journal of Diabetes Research 2016 (2016).. (Level of evidence: 1A) (last consulted on 05/12/2016)
Richard, J-L., M. Almasri, and S. Schuldiner. "Treatment of acute Charcot foot with bisphosphonates: a systematic review of the literature." Diabetologia 55.5 (2012): 1258-1264. (Level of evidence: 1A) (last consulted on 29/11/2016)
Gout[edit | edit source]
Min, Z., and M. Junwu. "Research progress in the genetics of hyperuricaemia and gout." Yi chuan= Hereditas/Zhongguo yi chuan xue hui bian ji 38.4 (2016): 300-313. (level of evidence: 1A) (last consulted on 5/12/16)
Harris, MARK D., LORI B. Siegel, and JEFFREY A. Alloway. "Gout and hyperuricemia." American family physician 59.4 (1999): 925-934. (level of evidence: 1A) (last consulted on 5/12/16)
Owens, D., B. Whelan, and G. McCarthy. "A survey of the management of gout in primary care." Irish medical journal 101.5 (2008): 147-149. (level of evidence: 1B) (last consulted on 5/12/16)
Borghi, C., and F. Perez-Ruiz. "Urate lowering therapies in the treatment of gout: a systematic review and meta-analysis." European review for medical and pharmacological sciences 20.5 (2016): 983-992. (level of evidence: 1A) (last consulted on 2/12/16)
Cronstein, Bruce N., and Robert Terkeltaub. "The inflammatory process of gout and its treatment." Arthritis Research & Therapy 8.1 (2006): 1. (level of evidence: 1A) (last consulted on 5/12/16)
Schlesinger, Naomi, et al. "Local ice therapy during bouts of acute gouty arthritis." The Journal of rheumatology 29.2 (2002): 331-334. (level of evidence: 1B) (last consulted on 5/12/16)
Lee, Won Bock, et al. "Acupuncture for gouty arthritis: a concise report of a systematic and meta-analysis approach." Rheumatology 52.7 (2013): 1225-1232. (level of evidence: 1A) (last consulted on 4/12/16)
Psoriatic arthritis[edit | edit source]
Mahmood, Farrouq, and Philip Helliwell. "PSORIATIC ARTHRITIS: A REVIEW." (Level of evidence: 1A) (Last consulted on 9/12/2016)
Amherd‐Hoekstra, Anne, et al. "Psoriatic arthritis: a review." JDDG: Journal der Deutschen Dermatologischen Gesellschaft 8.5 (2010): 332-339. (Level of evidence: 1A) (Last consulted on 3/12/2016)
Reactive arthritis[edit | edit source]
Olivieri I, Barozzi L, Padula A. Enthesiopathy: clinical manifestations, imaging and treatment. Baillieres Clin Rheumatol 1998;12(4). (Level of evidence: 1A) (Last consulted on 27/11/2016)
Kim, Paul S., Thomas L. Klausmeier, and Donald P. Orr. "Reactive arthritis: a review." Journal of Adolescent Health 44.4 (2009): 309-315. (Level of evidence: 1A) (Last consulted on 3/12/2016)
Silman, Alan J., and Marc C. Hochberg. Epidemiology of the rheumatic diseases. No. Ed. 2. Oxford University Press, 2001. (Level of evidence: 1A) (Last consulted on 9/12/2016)
Clinical Bottom Line[edit | edit source]
Ankle and Foot arthropathies is a very general subject. It covers all joint diseases of the foot and ankle. Our research showed that physical therapy can be effective in all cases of ankle and foot arthropathies we discussed. It is important to make a good differential diagnosis, so the right physical therapy management can be chosen for the specific disease.
References[edit | edit source]
- ↑ Reactive Arthritis (Reiter’s Syndrome). www.mayoclinic.org. Retrieved May 16, 2011.(accessed 3 december 2016)
- ↑ Björkengren A. G., Resnick D, Sartoris DJ. Enteropathic arthropathies. Radiologic Clinics of North America 1987: 189
- ↑ McGill, Neil W. Gout and other crystal-associated arthropathies. Best Practice & Research Clinical Rheumatology 2000: 445-460
- ↑ Medical diccionary. http://medical-dictionary.thefreedictionary.com/diabetic+arthropathy (Accessed 2 december 2016)
- ↑ Stauffer RN: Intra-articular ankle problems. In Evarts CM (ed): surgery of the musculoskeletal system, vol. 4. New York, Churchill-Livingstone, 1990.
- ↑ Huch, Klaus E. Kuettner, Dieppe P. Osteoarthritis in ankle and knee joints. Seminars in arthritis and rheumatism. 1997 Vol. 26. No. 4.
- ↑ Kerry RM, Holt GM, Stockley I. The foot in chronic rheumatoid arthritis: a continuing problem. The Foot 1994: 201-203.
- ↑ 8.0 8.1 8.2 8.3 8.4 Chan, Pui‐Shan J, Kok Ooi Kong. Natural history and imaging of subtalar and midfoot joint disease in rheumatoid arthritis. International journal of rheumatic diseases 2013;16: 14-18.
- ↑ Alehata, D., et al. Rheumatoid arthritis classification criteria: an American College of Rheumatology/European Union League Against Rheumatism collaborative initiative. Ann Rheum Dis 2010;69:1580-8.
- ↑ 10.0 10.1 10.2 10.3 Lohkamp M. et al. The prevalence of disabling foot pain in patients with early rheumatoid arthritis. The Foot 2006;16(4):201-207.
- ↑ 11.0 11.1 11.2 11.3 Brenton-Rule, Angela, et al. Foot and ankle characteristics associated with falls in adults with established rheumatoid arthritis: a cross-sectional study.BMC musculoskeletal disorders 2016;17(1):1.
- ↑ 12.0 12.1 12.2 12.3 12.4 Baillet, Athan, et al. Efficacy of resistance exercises in rheumatoid arthritis: meta-analysis of randomized controlled trials. Rheumatology 2012;51(3):519-527.
- ↑ 13.0 13.1 13.2 Barg, A., et al. Haemophilic arthropathy of the ankle treated by total ankle replacement: a case series. Haemophilia 2010;16(4):647-655.
- ↑ Fauzi, Aishah Ahmad, Chung Tze Yang.Bilateral diabetic Charcot foot. Australian family physician 2013;42:55.
- ↑ 15.0 15.1 15.2 15.3 15.4 15.5 Jeffcoate WJ. Charcot foot syndrome. Diabetic Medicine 2015;32(6):760-770.
- ↑ 16.0 16.1 16.2 Mabilleau, Guillaume, et al. Number of circulating CD14-positive cells and the serum levels of TNF-α are raised in acute charcot foot. Diabetes Care 2011;34(3):e33-e33.
- ↑ 17.0 17.1 17.2 Jeffcoate, William J, Fran Game, Peter R. Cavanagh. The role of proinflammatory cytokines in the cause of neuropathic osteoarthropathy (acute Charcot foot) in diabetes. The Lancet 2005;366(9502):2058-2061.
- ↑ 18.0 18.1 18.2 18.3 Smith, Caroline, Saravana Kumar, and Ryan Causby. The effectiveness of non‐surgical interventions in the treatment of Charcot foot. International Journal of Evidence‐Based Healthcare 2007;5(4):437-449.
- ↑ 19.00 19.01 19.02 19.03 19.04 19.05 19.06 19.07 19.08 19.09 19.10 19.11 19.12 19.13 Kucera, Tomas, Haroun Hassan Shaikh, and Pavel Sponer. Charcot Neuropathic Arthropathy of the Foot: A Literature Review and Single-Center Experience. Journal of Diabetes Research 2016
- ↑ Martillo, Miguel A., Lama Nazzal, Daria B, Crittenden. The crystallization of monosodium urate. Current rheumatology reports 2014;16(2):1-8.
- ↑ 21.00 21.01 21.02 21.03 21.04 21.05 21.06 21.07 21.08 21.09 21.10 21.11 Mahmood F, Helliwell P. Psoriatic Arthritis:a review.EMJ Rheumatol. 2016;3[1]:114-117.
- ↑ Coates L., Jaap F, Helliwell P. Defining minimal disease activity in psoriatic arthritis: a proposed objective target for treatment. Annals of the rheumatic diseases 2010;69(1):48-53.
- ↑ 23.0 23.1 23.2 23.3 23.4 23.5 Toivanen A , Toivanen P. Reactive arthritis. Best Practice & Research Clinical Rheumatology 2004;18(5): 689-703.
- ↑ 24.0 24.1 24.2 Kim PS., Klausmeier TL, Orr DP. Reactive arthritis: a review. Journal of Adolescent Health 2009;44(4):309-315.
- ↑ Roosendaal, G., and Lafeber FP. Pathogenesis of haemophilic arthropathy. Haemophilia 2006;12:117-121.
- ↑ 26.0 26.1 26.2 Kuo CF, et al. Global epidemiology of gout: prevalence, incidence and risk factors. Nature Reviews Rheumatology 2015;11(11):649-662.
- ↑ 27.0 27.1 Kim KY., et al. A literature review of the epidemiology and treatment of acute gout. Clinical therapeutics 2003;25(6):1593-1617.
- ↑ 28.0 28.1 28.2 28.3 28.4 28.5 28.6 28.7 Goodman CC, Fuller KS. Pathology: Implications for the Physical Therapist. 3rd ed. Saint Louis, MO: Saunders; 2009.
- ↑ Pascual, Eliseo. "Hyperuricemia and gout." Current opinion in rheumatology 6.4 (1994): 454.
- ↑ Min, Z., and M. Junwu. Research progress in the genetics of hyperuricaemia and gout. Yi chuan= Hereditas/Zhongguo yi chuan xue hui bian ji 2016;38(4):300-313.
- ↑ 31.0 31.1 31.2 Reach G. Treatment adherence in patients with gout. Joint Bone Spine 2011;78(5):456-459.
- ↑ Williams PT. Effects of diet, physical activity and performance, and body weight on incident gout in ostensibly healthy, vigorously active men. The American journal of clinical nutrition 2008;87(5):1480-1487.
- ↑ 33.0 33.1 Amherd‐Hoekstra A et al. Psoriatic arthritis: a review. JDDG: Journal der Deutschen Dermatologischen Gesellschaft 2010;8(5): 332-339.
- ↑ Mease P. Psoriatic arthritis update." BULLETIN-HOSPITAL FOR JOINT DISEASES NEW YORK 64.1/2 (2006): 25.
- ↑ 35.0 35.1 Klippel, John H., John H. Stone, and Patience H. White. Primer on the rheumatic diseases. Springer Science & Business Media, 2008.
- ↑ Kellgren J.H. 'Atlas of standard radiographs of arthritis'. Volume II of The Epidemiologic of Chronic Rheumatism. Oxford, Blackwell, 1963.
- ↑ Carroll M et al. Assessment of foot and ankle muscle strength using hand held dynamometry in patients with established rheumatoid arthritis. Journal of foot and ankle research 2013;6(1)
- ↑ Carroll M et al. Gait characteristics associated with the foot and ankle in inflammatory arthritis: a systematic review and meta-analysis. BMC musculoskeletal disorders 2015;16(1):1.
- ↑ Rongen‐van Dartel, S. A. A., et al. Effect of Aerobic Exercise Training on Fatigue in Rheumatoid Arthritis: A Meta‐Analysis.Arthritis care & research 2015;67(8): 1054-1062.
- ↑ 40.0 40.1 40.2 40.3 40.4 40.5 40.6 40.7 Leslie, Raffini, and Manno Catherine. Modern management of haemophilic arthropathy. British journal of haematology 2007;136(6): 777-787.
- ↑ 41.0 41.1 Lafeber FP., Miossec P, Valentino LA. Physiopathology of haemophilic arthropathy. Haemophilia 2008;14: 3-9
- ↑ 42.0 42.1 42.2 42.3 Shibata, T. O. H. R. U., K. Tada, and C. Hashizume. The results of arthrodesis of the ankle for leprotic neuroarthropathy. J Bone Joint Surg Am 1990; 72: 749-756.
- ↑ Harris, MARK D., LORI B. Siegel, and JEFFREY A. Alloway. Gout and hyperuricemia." American family physician 199;59(4):925-934.
- ↑ The Johns Hopkins University School of Medicine and the Johns Hopkins Arthritis Center.Psoriatic Arthritis. www.hopkinsmedicine.org/ (Accessed 27 november 2016
- ↑ http://www.arthritis.org/living-with-arthritis/tools-resources/expert-q-a/gout-questions/what-is-pseudogout.php (Accessed 3 december 16)
- ↑ 46.0 46.1 Wakefield, Richard J., et al. The value of sonography in the detection of bone erosions in patients with rheumatoid arthritis. Arthritis Rheum 2000;43(12):2762-70.
- ↑ 47.0 47.1 Lehtinen, Ari, et al. Painful ankle region in rheumatoid arthritis Analysis of soft-tissue changes with ultrasonography and MR imaging. Acta Radiologica 1996;37(3):572-577.
- ↑ 48.0 48.1 Doria, A. S. "State‐of‐the‐art imaging techniques for the evaluation of haemophilic arthropathy: present and future." Haemophilia 2010; 16:107-114.
- ↑ Wukich, D. K., et al. The consequences of complacency: managing the effects of unrecognized Charcot feet. Diabetic Medicine 2011;28(2):195-198.
- ↑ Kalish J, Hamdan A. Management of diabetic foot problems. Journal of vascular surgery 2010; 51(2):476-486.
- ↑ 51.0 51.1 Varma, Ajit Kumar. Charcot neuroarthropathy of the foot and ankle: a review. The journal of foot and ankle surgery 2013;52(6):740-749.
- ↑ Wallace, Stanley L., et al. Preliminary criteria for the classification of the acute arthritis of primary gout. Arthritis & Rheumatism 1977;20: 895-900.
- ↑ Owens D., Whelan B, McCarthy G. A survey of the management of gout in primary care. Irish medical journal 2008;101(5):147-149.
- ↑ Salk, Robert S., et al.Sodium hyaluronate in the treatment of osteoarthritis of the ankle: a controlled, randomized, double-blind pilot study. J Bone Joint Surg Am 2006;88(2):295-302.
- ↑ Domsic RT, Saltzman CL. Ankle osteoarthritis scale.Foot & ankle international 1998;19(7):466-471.
- ↑ 56.0 56.1 56.2 56.3 Gladman, Dafna D., et al. Outcome measures in psoriatic arthritis. The Journal of Rheumatology 2007;34(5):1159-1166.
- ↑ Dalbeth, Nicola, et al.Outcome measures in acute gout: a systematic literature review. The Journal of rheumatology 2014;41(3):558-568.
- ↑ Littlewood S. et al. Evaluation of a Psoriatic Arthritis Response Criteria Standardization Training Session for Clinicians. Rheumatology 2014;53(1):152.
- ↑ 59.0 59.1 59.2 59.3 59.4 D.M. Reid, C.G. Miller (eds.), Clinical Trials in Rheumatoid Arthritis and Osteoarthritis, Springer-Verlag London Limited 2008, 325p.
- ↑ 60.0 60.1 Witteveen, Angelique GH, Cheriel J. Hofstad, and Gino MMJ Kerkhoffs. "Hyaluronic acid and other conservative treatment options for osteoarthritis of the ankle." The Cochrane Library (2015)
- ↑ Roth, Sanford H., et al. Around-the-clock, controlled-release oxycodone therapy for osteoarthritis-related pain: placebo-controlled trial and long-term evaluation. Archives of Internal Medicine 2000;160(6):853-860.
- ↑ 62.0 62.1 62.2 62.3 Karatosun, V., et al. Intra-articular hyaluronic acid compared to exercise therapy in osteoarthritis of the ankle. A prospective randomized trial with long-term follow-up. Clinical and experimental rheumatology 2008; 26(2):288.
- ↑ Tanaka, Y., et al. "Low tibial osteotomy for varus-type osteoarthritis of the ankle." Bone & Joint Journal 88.7 (2006): 909-913.
- ↑ Ogilvie-Harris DJ, Sekyi-Otu A. Arthroscopic debridement for the osteoarthritic ankle. Arthroscopy: The Journal of Arthroscopic & Related Surgery 1995;11(4):433-436.
- ↑ Saltzman, Charles L., Robert G. Kadoko, and Jin Soo Suh. Treatment of isolated ankle osteoarthritis with arthrodesis or the total ankle replacement: a comparison of early outcomes.Clinics in orthopedic surgery 2010;2(1):1-7.
- ↑ D.T. Felson et al., Osteoarthritis : New inights, part 2: treatment approach, (level of evidence: 2A) (last consulted on 20/11/2016)
- ↑ Murosaki, Takamasa, et al. "Foot ulcers caused by rheumatoid vasculitis in a patient with rheumatoid arthritis undergoing etanercept treatment." Internal Medicine 51.22 (2012): 3181-3183.
- ↑ Graudal, Niels, et al. "Combination Therapy With and Without Tumor Necrosis Factor Inhibitors in Rheumatoid Arthritis: A Meta‐Analysis of Randomized Trials." Arthritis care & research 67.11 (2015): 1487-1495.
- ↑ Choy, Ernest H., et al. "A two year randomised controlled trial of intramuscular depot steroids in patients with established rheumatoid arthritis who have shown an incomplete response to disease modifying antirheumatic drugs." Annals of the rheumatic diseases 64.9 (2005): 1288-1293.
- ↑ Richard, J-L., M. Almasri, and S. Schuldiner. "Treatment of acute Charcot foot with bisphosphonates: a systematic review of the literature." Diabetologia 55.5 (2012): 1258-1264.
- ↑ Borghi, C., and F. Perez-Ruiz. "Urate lowering therapies in the treatment of gout: a systematic review and meta-analysis." European review for medical and pharmacological sciences 20.5 (2016): 983-992.
- ↑ 72.0 72.1 72.2 72.3 72.4 Cronstein, Bruce N., and Robert Terkeltaub. "The inflammatory process of gout and its treatment." Arthritis Research & Therapy 8.1 (2006): 1.
- ↑ 73.0 73.1 Fryden, Aril, et al. "Early antibiotic treatment of reactive arthritis associated with enteric infections: clinical and serological study." BMJ 301.6764 (1990): 1299-1302.
- ↑ Palazzi, Carlo, et al. "Management of reactive arthritis." Expert opinion on pharmacotherapy 5.1 (2004): 61-70.
- ↑ van der Linden, Sjef, and Désirée van der Heijde. "Clinical aspects, outcome assessment, and management of ankylosing spondylitis and postenteric reactive arthritis." Current opinion in rheumatology 12.4 (2000): 263-268.
- ↑ Mielants, H., et al. "HLA-B27 related arthritis and bowel inflammation. Part 2. Ileocolonoscopy and bowel histology in patients with HLA-B27 related arthritis." The Journal of rheumatology 12.2 (1985): 294-298.
- ↑ 77.0 77.1 Palazzi, Carlo, et al. "Management of reactive arthritis." Expert opinion on pharmacotherapy 5.1 (2004): 61-70.
- ↑ 78.0 78.1 Scott, A., Gidlow, C., Clinical exercise science, Taylor & Francis Ltd, 2016
- ↑ D.T. Felson et al., Osteoarthritis : New inights, part 2: treatment approach
- ↑ Lemmey, Andrew B., et al. "Are the benefits of a high‐intensity progressive resistance training program sustained in rheumatoid arthritis patients? A 3‐year followup study." Arthritis care & research 64.1 (2012): 71-75.
- ↑ Cho, Nam Soon, et al. "Randomized controlled trial for clinical effects of varying types of insoles combined with specialized shoes in patients with rheumatoid arthritis of the foot." Clinical rehabilitation 23.6 (2009/): 512-521.
- ↑ Woodburn, James, Sharon Barker, and Philip S. Helliwell. "A randomized controlled trial of foot orthoses in rheumatoid arthritis." The Journal of rheumatology 29.7 (2002): 1377-1383.
- ↑ 83.0 83.1 Nagel, Arne, Henrik Schmiegel Andreas Lars, and Dieter Rosenbaum. "Long-term effects of orthotic insoles in rheumatoid arthritis—A one-year year longitudinal follow-up." Gait & Posture 24 (2006): S178-S179.
- ↑ Mejjad, Othmane, et al. "Foot orthotics decrease pain but do not improve gait in rheumatoid arthritis patients." Joint Bone Spine 71.6 (2004): 542-545.
- ↑ Conceição, Cristiano Sena da, et al. "Systematic review and meta-analysis of effects of foot orthoses on pain and disability in rheumatoid arthritis patients." Disability and rehabilitation 37.14 (2015): 1209-1213.
- ↑ 86.0 86.1 Cuesta-Barriuso, Rubén, Antonia Gómez-Conesa, and José-Antonio López-Pina. "Manual therapy in the treatment of ankle hemophilic arthropathy. A randomized pilot study." Physiotherapy theory and practice 30.8 (2014): 534-539.
- ↑ 87.0 87.1 Lobet, Sébastien, et al. "Functional impact of custom‐made foot orthoses in patients with haemophilic ankle arthropathy." Haemophilia 18.3 (2012): e227-e235.
- ↑ 88.0 88.1 Kalish, Jeffrey, and Allen Hamdan. "Management of diabetic foot problems." Journal of vascular surgery 51.2 (2010): 476-486.
- ↑ R.T. Crews, J.S. Wrobel, Physical management of the Charcot foot, Clin Podiatr Med Surg, Volume 25, Issue 1; Pages 71-9, vii, 2008.
- ↑ Schlesinger, Naomi, et al. "Local ice therapy during bouts of acute gouty arthritis." The Journal of rheumatology 29.2 (2002): 331-334.
- ↑ Lee, Won Bock, et al. "Acupuncture for gouty arthritis: a concise report of a systematic and meta-analysis approach." Rheumatology 52.7 (2013): 1225-1232.
- ↑ 92.0 92.1 92.2 http://www.physio-pedia.com/Reiter's_Syndrome#Physical_Therapy_Management_.28 current_best_evidence.29
- ↑ Verbruggen L. Reumatologie, Reactieve arthritis. Vrije Universiteit Brussel, 3de Bachelor REVAKI 2011;48-51.
- ↑ Koehler L, Kuipers JG, Zeidler H. Managing seronegative spondarthritides. Rheumatol 2000;39:360-368.
- ↑ Olivieri I, Barozzi L, Padula A. Enthesiopathy: clinical manifestations, imaging and treatment. Baillieres Clin Rheumatol 1998;12(4).